Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells
- PMID: 23620405
- PMCID: PMC3804130
- DOI: 10.1158/1078-0432.CCR-13-0330
Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells
Abstract
Purpose: The adoptive transfer of T cells modified to express a chimeric antigen receptor (CAR) comprised of an extracellular single-chain antibody (scFV) fragment specific for a tumor cell surface molecule, and linked to an intracellular signaling module, has activity in advanced malignancies. The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a tumor-associated molecule expressed in prevalent B-lymphoid and epithelial cancers and is absent on normal mature B cells and vital tissues, making it a candidate for CAR T-cell therapy.
Experimental design: We constructed ROR1-CARs from scFVs with different affinities and containing extracellular IgG4-Fc spacer domains of different lengths, and evaluated the ability of T cells expressing each CAR to recognize ROR1(+) hematopoietic and epithelial tumors in vitro, and to eliminate human mantle cell lymphoma (MCL) engrafted into immunodeficient mice.
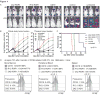
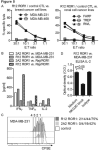
Results: ROR1-CARs containing a short "Hinge-only" extracellular spacer conferred superior lysis of ROR1(+) tumor cells and induction of T-cell effector functions compared with CARs with long "Hinge-CH2-CH3" spacers. CARs derived from a higher affinity scFV conferred maximum T-cell effector function against primary CLL and ROR1(+) epithelial cancer lines in vitro without inducing activation-induced T-cell death. T cells modified with an optimal ROR1-CAR were equivalently effective as CD19-CAR-modified T cells in mediating regression of JeKo-1 MCL in immunodeficient mice.
Conclusions: Our results show that customizing spacer design and increasing affinity of ROR1-CARs enhances T-cell effector function and recognition of ROR1(+) tumors. T cells modified with an optimized ROR1-CAR have significant antitumor efficacy in a preclinical model in vivo, suggesting they may be useful to treat ROR1(+) tumors in clinical applications.
Conflict of interest statement
Figures

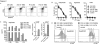
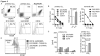
Similar articles
-
The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor.Blood. 2010 Nov 25;116(22):4532-41. doi: 10.1182/blood-2010-05-283309. Epub 2010 Aug 11. Blood. 2010. PMID: 20702778 Free PMC article.
-
Sleeping Beauty Transposition of Chimeric Antigen Receptors Targeting Receptor Tyrosine Kinase-Like Orphan Receptor-1 (ROR1) into Diverse Memory T-Cell Populations.PLoS One. 2015 Jun 1;10(6):e0128151. doi: 10.1371/journal.pone.0128151. eCollection 2015. PLoS One. 2015. PMID: 26030772 Free PMC article.
-
The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity.Cancer Immunol Res. 2015 Feb;3(2):125-35. doi: 10.1158/2326-6066.CIR-14-0127. Epub 2014 Sep 11. Cancer Immunol Res. 2015. PMID: 25212991 Free PMC article.
-
Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells.Immunol Rev. 2014 Jan;257(1):127-44. doi: 10.1111/imr.12139. Immunol Rev. 2014. PMID: 24329794 Free PMC article. Review.
-
From therapeutic antibodies to chimeric antigen receptors (CARs): making better CARs based on antigen-binding domain.Expert Opin Biol Ther. 2016 Dec;16(12):1469-1478. doi: 10.1080/14712598.2016.1235148. Epub 2016 Sep 19. Expert Opin Biol Ther. 2016. PMID: 27618260 Review.
Cited by
-
Inhibitory CARs fail to protect from immediate T cell cytotoxicity.Mol Ther. 2024 Apr 3;32(4):982-999. doi: 10.1016/j.ymthe.2024.02.022. Epub 2024 Feb 22. Mol Ther. 2024. PMID: 38384128 Free PMC article.
-
Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model.Gene Ther. 2015 May;22(5):391-403. doi: 10.1038/gt.2015.4. Epub 2015 Feb 5. Gene Ther. 2015. PMID: 25652098
-
Nonsignaling extracellular spacer regulates tumor antigen selectivity of CAR T cells.Mol Ther Oncol. 2024 Mar 2;32(2):200789. doi: 10.1016/j.omton.2024.200789. eCollection 2024 Jun 20. Mol Ther Oncol. 2024. PMID: 38939825 Free PMC article.
-
Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy.Clin Cancer Res. 2016 Apr 15;22(8):1875-84. doi: 10.1158/1078-0432.CCR-15-1433. Clin Cancer Res. 2016. PMID: 27084741 Free PMC article. Review.
-
Engineering CAR-T cells: Design concepts.Trends Immunol. 2015 Aug;36(8):494-502. doi: 10.1016/j.it.2015.06.004. Epub 2015 Jul 11. Trends Immunol. 2015. PMID: 26169254 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

