Analysis of SNARE complex/synaptotagmin-1 interactions by one-dimensional NMR spectroscopy
- PMID: 23617808
- PMCID: PMC3812274
- DOI: 10.1021/bi400230u
Analysis of SNARE complex/synaptotagmin-1 interactions by one-dimensional NMR spectroscopy
Abstract
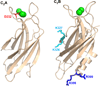
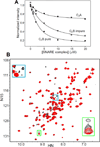
Neurotransmitter release depends critically on the Ca(2+) sensor synaptotagmin-1 and the SNARE proteins syntaxin-1, synaptobrevin, and SNAP-25, which mediate membrane fusion by forming tight SNARE complexes that bridge the synaptic vesicle and plasma membranes. Interactions between the SNARE complex and the two C2 domains of synaptotagmin-1 (the C2A and C2B domains) are believed to play a key role in coupling Ca(2+) sensing to membrane fusion, but the nature of these interactions is unclear, in part because of a paucity of data obtained by quantitative biophysical methods. Here we have analyzed synaptotagmin-1/SNARE complex interactions by monitoring the decrease in the intensities of one-dimensional (13)C-edited (1)H NMR spectra of (13)C-labeled fragments of synaptotagmin-1 upon binding to unlabeled SNARE complex. Our results indicate that there is a primary binding mode between synaptotagmin-1 and the SNARE complex that involves a polybasic region in the C2B domain and has a sub-micromolar affinity. Our NMR data, combined with precipitation assays, show that there are additional SNARE complex/synaptotagmin-1 interactions that lead to aggregation and that involve in part two arginines at the bottom of the C2B domain. Overall, this study shows the importance of disentangling the contributions of different types of interactions to SNARE complex/synaptotagmin-1 binding and illustrates the usefulness of one-dimensional NMR methods to analyze intricate protein interactions.
Figures






Similar articles
-
Polybasic Patches in Both C2 Domains of Synaptotagmin-1 Are Required for Evoked Neurotransmitter Release.J Neurosci. 2022 Jul 27;42(30):5816-5829. doi: 10.1523/JNEUROSCI.1385-21.2022. Epub 2022 Jun 14. J Neurosci. 2022. PMID: 35701163 Free PMC article.
-
Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution.Nat Struct Mol Biol. 2015 Jul;22(7):555-64. doi: 10.1038/nsmb.3035. Epub 2015 Jun 1. Nat Struct Mol Biol. 2015. PMID: 26030874 Free PMC article.
-
Ca2+-dependent release of synaptotagmin-1 from the SNARE complex on phosphatidylinositol 4,5-bisphosphate-containing membranes.Elife. 2020 Aug 18;9:e57154. doi: 10.7554/eLife.57154. Elife. 2020. PMID: 32808925 Free PMC article.
-
Enlightening molecular mechanisms through study of protein interactions.J Mol Cell Biol. 2012 Oct;4(5):270-83. doi: 10.1093/jmcb/mjs036. Epub 2012 Jun 26. J Mol Cell Biol. 2012. PMID: 22735643 Free PMC article. Review.
-
Synaptic vesicle fusion.Nat Struct Mol Biol. 2008 Jul;15(7):665-74. doi: 10.1038/nsmb.1450. Nat Struct Mol Biol. 2008. PMID: 18618940 Free PMC article. Review.
Cited by
-
Evaluation of the tert-butyl group as a probe for NMR studies of macromolecular complexes.J Biomol NMR. 2021 Sep;75(8-9):347-363. doi: 10.1007/s10858-021-00380-y. Epub 2021 Sep 9. J Biomol NMR. 2021. PMID: 34505210 Free PMC article.
-
Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment.Nat Neurosci. 2018 Jan;21(1):33-40. doi: 10.1038/s41593-017-0037-5. Epub 2017 Dec 11. Nat Neurosci. 2018. PMID: 29230057 Free PMC article.
-
Mechanism of neurotransmitter release coming into focus.Protein Sci. 2018 Aug;27(8):1364-1391. doi: 10.1002/pro.3445. Epub 2018 Jul 10. Protein Sci. 2018. PMID: 29893445 Free PMC article. Review.
-
Correlation between evoked neurotransmitter release and adaptive functions in SYT1-associated neurodevelopmental disorder.EBioMedicine. 2024 Nov;109:105416. doi: 10.1016/j.ebiom.2024.105416. Epub 2024 Oct 30. EBioMedicine. 2024. PMID: 39481209 Free PMC article.
-
Analysis of tripartite Synaptotagmin-1-SNARE-complexin-1 complexes in solution.FEBS Open Bio. 2023 Jan;13(1):26-50. doi: 10.1002/2211-5463.13503. Epub 2022 Nov 16. FEBS Open Bio. 2023. PMID: 36305864 Free PMC article.
References
-
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q.Rev. Biophys. 2005:1–47. - PubMed
-
- Sorensen JB. Conflicting views on the membrane fusion machinery and the fusion pore. Annu.Rev. Cell Dev. Biol. 2009;25:513–537. - PubMed
-
- Rizo J, Sudhof TC. The Membrane Fusion Enigma: SNAREs, Sec1/Munc18 Proteins, and Their Accomplices-Guilty as Charged? Annu.Rev. Cell Dev. Biol. 2012;28:279–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

