Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis
- PMID: 23613806
- PMCID: PMC3629222
- DOI: 10.1371/journal.pone.0061177
Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis
Abstract
Objectives: Ceftriaxone increases expression of the astrocytic glutamate transporter, EAAT2, which might protect from glutamate-mediated excitotoxicity. A trial using a novel three stage nonstop design, incorporating Phases I-III, tested ceftriaxone in ALS. Stage 1 determined the cerebrospinal fluid pharmacokinetics of ceftriaxone in subjects with ALS. Stage 2 evaluated safety and tolerability for 20-weeks. Analysis of the pharmacokinetics, tolerability, and safety was used to determine the ceftriaxone dosage for Stage 3 efficacy testing.
Methods: In Stage 1, 66 subjects at ten clinical sites were enrolled and randomized equally into three study groups receiving intravenous placebo, ceftriaxone 2 grams daily or ceftriaxone 4 grams daily divided BID. Participants provided serum and cerebrospinal fluid for pharmacokinetic analysis on study day 7. Participants continued their assigned treatment in Stage 2. The Data and Safety Monitoring Board (DSMB) reviewed the data after the last participants completed 20 weeks on study drug.
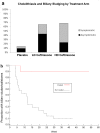
Results: Stage 1 analysis revealed linear pharmacokinetics, and CSF trough levels for both dosage levels exceeding the pre-specified target trough level of 1 µM (0.55 µg/mL). Tolerability (Stages 1 and 2) results showed that ceftriaxone at dosages up to 4 grams/day was well tolerated at 20 weeks. Biliary adverse events were more common with ceftriaxone but not dose-dependent and improved with ursodeoxycholic (ursodiol) therapy.
Conclusions: The goals of Stages 1 and 2 of the ceftriaxone trial were successfully achieved. Based on the pre-specified decision rules, the DSMB recommended the use of ceftriaxone 4 g/d (divided BID) for Stage 3, which recently closed.
Trial registration: ClinicalTrials.gov NCT00349622.
Conflict of interest statement
Figures
Similar articles
-
Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial.Lancet Neurol. 2014 Nov;13(11):1083-1091. doi: 10.1016/S1474-4422(14)70222-4. Epub 2014 Oct 5. Lancet Neurol. 2014. PMID: 25297012 Free PMC article. Clinical Trial.
-
Systemic pharmacokinetics and cerebrospinal fluid uptake of intravenous ceftriaxone in patients with amyotrophic lateral sclerosis.J Clin Pharmacol. 2014 Oct;54(10):1180-7. doi: 10.1002/jcph.317. Epub 2014 May 16. J Clin Pharmacol. 2014. PMID: 24771634 Free PMC article. Clinical Trial.
-
Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis.Clin Neuropharmacol. 2010 Jan-Feb;33(1):17-21. doi: 10.1097/WNF.0b013e3181c47569. Clin Neuropharmacol. 2010. PMID: 19935406 Clinical Trial.
-
Safety and tolerability of ertapenem.J Antimicrob Chemother. 2004 Jun;53 Suppl 2:ii75-81. doi: 10.1093/jac/dkh209. J Antimicrob Chemother. 2004. PMID: 15150186 Review.
-
Predicting success: optimizing phase II ALS trials for the transition to phase III.Amyotroph Lateral Scler Frontotemporal Degener. 2014 Mar;15(1-2):1-8. doi: 10.3109/21678421.2013.838969. Amyotroph Lateral Scler Frontotemporal Degener. 2014. PMID: 24588460 Review.
Cited by
-
Membrane transporters in traumatic brain injury: Pathological, pharmacotherapeutic, and developmental implications.Exp Neurol. 2019 Jul;317:10-21. doi: 10.1016/j.expneurol.2019.02.011. Epub 2019 Feb 21. Exp Neurol. 2019. PMID: 30797827 Free PMC article. Review.
-
Repurposing of the β-Lactam Antibiotic, Ceftriaxone for Neurological Disorders: A Review.Front Neurosci. 2019 Mar 26;13:236. doi: 10.3389/fnins.2019.00236. eCollection 2019. Front Neurosci. 2019. PMID: 30971875 Free PMC article. Review.
-
Overcoming Drug Development Bottlenecks With Repurposing: Old drugs learn new tricks.Nat Med. 2014 Jun;20(6):590-1. doi: 10.1038/nm.3595. Nat Med. 2014. PMID: 24901567 Free PMC article. No abstract available.
-
The Glt1 glutamate receptor mediates the establishment and perpetuation of chronic visceral pain in an animal model of stress-induced bladder hyperalgesia.Am J Physiol Renal Physiol. 2016 Apr 1;310(7):F628-F636. doi: 10.1152/ajprenal.00297.2015. Epub 2015 Dec 23. Am J Physiol Renal Physiol. 2016. PMID: 26697981 Free PMC article.
-
Targeting for Success: Demonstrating Proof-of-Concept with Mechanistic Early Phase Clinical Pharmacology Studies for Disease-Modification in Neurodegenerative Disorders.Int J Mol Sci. 2021 Feb 5;22(4):1615. doi: 10.3390/ijms22041615. Int J Mol Sci. 2021. PMID: 33562713 Free PMC article. Review.
References
-
- Worms PM (2001) The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci 191: 3–9. - PubMed
-
- Haverkamp LJ, Appel V, Appel SH (1995) Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 118 (Pt 3): 707–719. - PubMed
-
- Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 330: 585–591. - PubMed
-
- Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 3: CD001447. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

