Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy
- PMID: 23612193
- PMCID: PMC3697338
- DOI: 10.1128/AAC.00267-13
Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy
Abstract
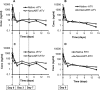
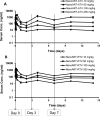
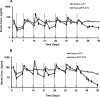
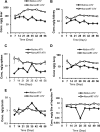
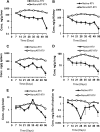
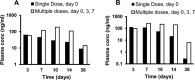
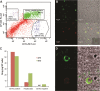
Long-acting injectable nanoformulated antiretroviral therapy (nanoART) was developed with the explicit goal of improving medicine compliance and for drug targeting of viral tissue reservoirs. Prior nanoART studies completed in humanized virus-infected mice demonstrated sustained antiretroviral responses. However, the pharmacokinetics (PK) and tissue distribution of nanoART were not characterized. To this end, the PK and tissue distribution of nanoformulated atazanavir (ATV) and ritonavir (RTV) injected subcutaneously or intramuscularly in mice and monkeys were evaluated. Fourteen days after injection, ATV and RTV levels were up to 13-, 41-, and 4,500-fold higher than those resulting from native-drug administration in plasma, tissues, and at the site of injection, respectively. At nanoART doses of 10, 50, 100, and 250 mg/kg of body weight, relationships of more- and less-than-proportional increases in plasma and tissue levels with dose increases were demonstrated with ATV and RTV. Multiple-dose regimens showed serum and tissue concentrations up to 270-fold higher than native-drug concentrations throughout 8 weeks of study. Importantly, nanoART was localized in nonlysosomal compartments in tissue macrophages, creating intracellular depot sites. Reflective data were obtained in representative rhesus macaque studies. We conclude that nanoART demonstrates blood and tissue antiretroviral drug levels that are enhanced compared to those of native drugs. The sustained and enhanced PK profile of nanoART is, at least in part, the result of the sustained release of ATV and RTV from tissue macrophases and at the site of injection.
Figures
Similar articles
-
Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections.Nanomedicine. 2013 Nov;9(8):1263-73. doi: 10.1016/j.nano.2013.05.003. Epub 2013 May 13. Nanomedicine. 2013. PMID: 23680933 Free PMC article.
-
Pharmacokinetics, biodistribution, and toxicity of folic acid-coated antiretroviral nanoformulations.Antimicrob Agents Chemother. 2014 Dec;58(12):7510-9. doi: 10.1128/AAC.04108-14. Epub 2014 Oct 6. Antimicrob Agents Chemother. 2014. PMID: 25288084 Free PMC article.
-
Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice.AIDS. 2012 Nov 13;26(17):2135-44. doi: 10.1097/QAD.0b013e328357f5ad. AIDS. 2012. PMID: 22824628 Free PMC article.
-
Atazanavir: in pediatric patients with HIV-1 infection.Paediatr Drugs. 2012 Apr 1;14(2):131-41. doi: 10.2165/11208550-000000000-00000. Paediatr Drugs. 2012. PMID: 22292486 Review.
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
Cited by
-
The Synergistic Effect of an ATP-Competitive Inhibitor of mTOR and Metformin on Pancreatic Tumor Growth.Curr Dev Nutr. 2020 Aug 10;4(9):nzaa131. doi: 10.1093/cdn/nzaa131. eCollection 2020 Sep. Curr Dev Nutr. 2020. PMID: 32908958 Free PMC article.
-
mTORC1 and mTORC2 Complexes Regulate the Untargeted Metabolomics and Amino Acid Metabolites Profile through Mitochondrial Bioenergetic Functions in Pancreatic Beta Cells.Nutrients. 2022 Jul 22;14(15):3022. doi: 10.3390/nu14153022. Nutrients. 2022. PMID: 35893876 Free PMC article.
-
Macrophage Targeted Nanoparticles for Antiretroviral (ARV) Delivery.J Pers Nanomed. 2015 Nov;1(2):40-48. Epub 2015 Nov 14. J Pers Nanomed. 2015. PMID: 29492319 Free PMC article.
-
Pharmacokinetics of a Long-Acting Nanoformulated Dolutegravir Prodrug in Rhesus Macaques.Antimicrob Agents Chemother. 2017 Dec 21;62(1):e01316-17. doi: 10.1128/AAC.01316-17. Print 2018 Jan. Antimicrob Agents Chemother. 2017. PMID: 29061742 Free PMC article.
-
Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production.Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00825-20. doi: 10.1128/AAC.00825-20. Print 2020 Sep 21. Antimicrob Agents Chemother. 2020. PMID: 32759267 Free PMC article.
References
-
- Ellis R, Langford D, Masliah E. 2007. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat. Rev. Neurosci. 8:33–44 - PubMed
-
- Work Group for HIV and Aging Consensus Project 2012. Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. J. Am. Geriatr. Soc. 60:974–979 - PubMed
-
- Pasternak AO, de Bruin M, Jurriaans S, Bakker M, Berkhout B, Prins JM, Lukashov VV. 2012. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J. Infect. Dis. 206:1443–1452 - PubMed
-
- Coiras M, Lopez-Huertas MR, Alcami J. 2010. HIV-1 latency and eradication of long-term viral reservoirs. Discov. Med. 9:185–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- P01 DA026146/DA/NIDA NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R01 NS36126/NS/NINDS NIH HHS/United States
- DA028555-01/DA/NIDA NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P20 DA026146/DA/NIDA NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P20 RR15635/RR/NCRR NIH HHS/United States
- R01 NS070190/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

