Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes
- PMID: 23611899
- PMCID: PMC3766901
- DOI: 10.1016/j.joca.2013.04.011
Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes
Abstract
Objective: Pro-inflammatory cytokines play an important role in inducing cartilage degradation during osteoarthritis pathogenesis. Muscle is a tissue that lies near cartilage in situ. However, muscle's non-loading biochemical effect on cartilage has been largely unexplored. Here, we tested the hypothesis that muscle cells can regulate the response to pro-inflammatory cytokine-mediated damage in chondrocytes derived from human bone marrow-derived mesenchymal stem cells (hMSCs).
Method: hMSCs were allowed to undergo chondrogenic differentiation in porous silk scaffolds in the typical chondrogenic medium for 12 days. For the next 9 days, the cells were cultured in chondrogenic medium containing 50% conditioned medium derived from C2C12 muscle cells or fibroblast control cells, and were subject to treatments of pro-inflammatory cytokines IL-1β or TNFα.
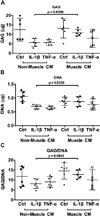
Results: Both IL-1β and TNFα-induced strong expression of multiple MMPs and hypertrophic markers Runx2 and type X collagen. Strikingly, culturing hMSC-derived chondrocytes in C2C12 muscle cell-conditioned medium strongly inhibited the expression of all these genes, a result further confirmed by GAG content and histological evaluation of matrix protein. To determine whether these effects were due to altered chondrocyte growth and survival, we assayed the expression of cell proliferation marker Ki67, cell cycle arrest markers p21 and p53, and apoptosis marker caspase 3. Muscle cell-conditioned medium promoted proliferation and inhibited apoptosis, thereby suggesting a possible decrease in the cellular aging and death that typically accompanies cartilage inflammation.
Conclusion: Our findings suggest the role of muscle in cartilage homeostasis and provide insight into designing strategies for promoting resistance to pro-inflammatory cytokines in hMSC-derived chondrocytes.
Copyright © 2013 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors have any conflict of interest related to this work.
Figures




Similar articles
-
The Therapeutic Potential of Adipose-Derived Mesenchymal Stem Cell Secretome in Osteoarthritis: A Comprehensive Study.Int J Mol Sci. 2024 Oct 20;25(20):11287. doi: 10.3390/ijms252011287. Int J Mol Sci. 2024. PMID: 39457070 Free PMC article.
-
Synovium stem cell-derived matrix enhances anti-inflammatory properties of rabbit articular chondrocytes via the SIRT1 pathway.Mater Sci Eng C Mater Biol Appl. 2020 Jan;106:110286. doi: 10.1016/j.msec.2019.110286. Epub 2019 Oct 7. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31753397 Free PMC article.
-
Muscle cells enhance resistance to pro-inflammatory cytokine-induced cartilage destruction.Biochem Biophys Res Commun. 2010 Jan 29;392(1):22-8. doi: 10.1016/j.bbrc.2009.12.138. Epub 2009 Dec 31. Biochem Biophys Res Commun. 2010. PMID: 20043873 Free PMC article.
-
Cyanidin suppresses autophagic activity regulating chondrocyte hypertrophic differentiation.J Cell Physiol. 2018 Mar;233(3):2332-2342. doi: 10.1002/jcp.26105. Epub 2017 Aug 25. J Cell Physiol. 2018. PMID: 28722162
-
Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells.Eur Cell Mater. 2012 Jul 24;24:118-35; discussion 135. doi: 10.22203/ecm.v024a09. Eur Cell Mater. 2012. PMID: 22828990 Review.
Cited by
-
Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy.Biomedicines. 2023 Aug 10;11(8):2244. doi: 10.3390/biomedicines11082244. Biomedicines. 2023. PMID: 37626740 Free PMC article. Review.
-
Trajectories and Predictors of Weight-Bearing and Non-Weight-Bearing Pain in Knee Osteoarthritis: A 9-Year Follow-Up Study.J Pain Res. 2024 Oct 16;17:3385-3395. doi: 10.2147/JPR.S480910. eCollection 2024. J Pain Res. 2024. PMID: 39429515 Free PMC article.
-
Anti-inflammatory strategies in cartilage repair.Tissue Eng Part B Rev. 2014 Dec;20(6):655-68. doi: 10.1089/ten.TEB.2014.0014. Epub 2014 Jun 23. Tissue Eng Part B Rev. 2014. PMID: 24846478 Free PMC article. Review.
-
TNFα-Related Chondrocyte Inflammation Models: A Systematic Review.Int J Mol Sci. 2024 Oct 8;25(19):10805. doi: 10.3390/ijms251910805. Int J Mol Sci. 2024. PMID: 39409134 Free PMC article.
-
Intercellular communication via gap junction channels between chondrocytes and bone cells.Biochim Biophys Acta Biomembr. 2018 Dec;1860(12):2499-2505. doi: 10.1016/j.bbamem.2018.09.009. Epub 2018 Sep 14. Biochim Biophys Acta Biomembr. 2018. PMID: 30279151 Free PMC article.
References
-
- Little CJ, Bawolin NK, Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B Rev. 2011 Aug;17(4):213–227. - PubMed
-
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006 Jan;54(1):226–229. - PubMed
-
- Mahmoudifar N, Doran PM. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol. 2012 Mar;30(3):166–176. - PubMed
-
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis e an untreatable disease? Nat Rev Drug Discov. 2005 Apr;4(4):331–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

