RGD capsid modification enhances mucosal protective immunity of a non-human primate adenovirus vector expressing Pseudomonas aeruginosa OprF
- PMID: 23607394
- PMCID: PMC3722923
- DOI: 10.1111/cei.12101
RGD capsid modification enhances mucosal protective immunity of a non-human primate adenovirus vector expressing Pseudomonas aeruginosa OprF
Abstract
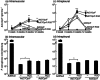
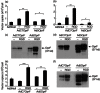
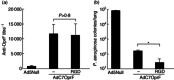
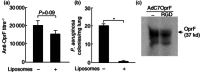
Replication-deficient adenoviral (Ad) vectors of non-human serotypes can serve as Ad vaccine platforms to circumvent pre-existing anti-human Ad immunity. We found previously that, in addition to that feature, a non-human primate-based AdC7 vector expressing outer membrane protein F of P. aeruginosa (AdC7OprF) was more potent in inducing lung mucosal and protective immunity compared to a human Ad5-based vector. In this study we analysed if genetic modification of the AdC7 fibre to display an integrin-binding arginine-glycine-aspartic acid (RGD) sequence can further enhance lung mucosal immunogenicity of AdC7OprF. Intratracheal immunization of mice with either AdC7OprF.RGD or AdC7OprF induced robust serum levels of anti-OprF immunoglobulin (Ig)G up to 12 weeks that were higher compared to immunization with the human vectors Ad5OprF or Ad5OprF.RGD. OprF-specific cellular responses in lung T cells isolated from mice immunized with AdC7OprF.RGD and AdC7OprF were similar for T helper type 1 (Th1) [interferon (IFN)-γ in CD8(+) and interleukin (IL)-12 in CD4(+)], Th2 (IL-4, IL-5 and IL-13 in CD4(+)) and Th17 (IL-17 in CD4(+)). Interestingly, AdC7OprF.RGD induced more robust protective immunity against pulmonary infection with P. aeruginosa compared to AdC7OprF or the control Ad5 vectors. The enhanced protective immunity induced by AdC7OprF.RGD was maintained in the absence of alveolar macrophages (AM) or CD1d natural killer T cells. Together, the data suggest that addition of RGD to the fibre of an AdC7-based vaccine is useful to enhance its mucosal protective immunogenicity.
Keywords: RGD; adenovirus; lung; mucosal immunity; pseudomonas.
© 2013 British Society for Immunology.
Figures

Similar articles
-
Post-exposure immunization by capsid-modified AdC7 vector expressing Pseudomonas aeruginosa OprF clears P. aeruginosa respiratory infection.Vaccine. 2017 Dec 18;35(51):7174-7180. doi: 10.1016/j.vaccine.2017.10.078. Epub 2017 Nov 7. Vaccine. 2017. PMID: 29126807 Free PMC article.
-
Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF.Vaccine. 2011 Mar 3;29(11):2131-9. doi: 10.1016/j.vaccine.2010.12.087. Epub 2011 Jan 6. Vaccine. 2011. PMID: 21215829 Free PMC article.
-
Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF.J Virol. 2007 Dec;81(24):13801-8. doi: 10.1128/JVI.01246-07. Epub 2007 Oct 17. J Virol. 2007. PMID: 17942539 Free PMC article.
-
Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid.J Clin Invest. 2005 May;115(5):1281-9. doi: 10.1172/JCI23135. Epub 2005 Apr 1. J Clin Invest. 2005. PMID: 15841217 Free PMC article.
-
Adenoviruses as vectors for delivering vaccines to mucosal surfaces.J Biotechnol. 2000 Sep 29;83(1-2):105-13. doi: 10.1016/s0168-1656(00)00314-x. J Biotechnol. 2000. PMID: 11000466 Free PMC article. Review.
Cited by
-
Simian adenoviruses as vaccine vectors.Future Virol. 2016 Sep;11(9):649-659. doi: 10.2217/fvl-2016-0070. Epub 2016 Sep 15. Future Virol. 2016. PMID: 29527232 Free PMC article. Review.
-
Post-exposure immunization by capsid-modified AdC7 vector expressing Pseudomonas aeruginosa OprF clears P. aeruginosa respiratory infection.Vaccine. 2017 Dec 18;35(51):7174-7180. doi: 10.1016/j.vaccine.2017.10.078. Epub 2017 Nov 7. Vaccine. 2017. PMID: 29126807 Free PMC article.
-
Hitting the nail on the head: combining oncolytic adenovirus-mediated virotherapy and immunomodulation for the treatment of glioma.Oncotarget. 2017 Sep 11;8(51):89391-89405. doi: 10.18632/oncotarget.20810. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179527 Free PMC article. Review.
-
Vaccines for Pseudomonas aeruginosa: a long and winding road.Expert Rev Vaccines. 2014 Apr;13(4):507-19. doi: 10.1586/14760584.2014.890053. Epub 2014 Feb 27. Expert Rev Vaccines. 2014. PMID: 24575895 Free PMC article. Review.
-
Identification of OprF as a complement component C3 binding acceptor molecule on the surface of Pseudomonas aeruginosa.Infect Immun. 2015 Aug;83(8):3006-14. doi: 10.1128/IAI.00081-15. Epub 2015 May 11. Infect Immun. 2015. PMID: 25964476 Free PMC article.
References
-
- Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Infect Control. 2006;34:S3–10. - PubMed
-
- Doring G, Pier GB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine. 2008;26:1011–1024. - PubMed
-
- Holder IA. Pseudomonas immunotherapy: a historical overview. Vaccine. 2004;22:831–839. - PubMed
-
- Sedlak-Weinstein E, Cripps AW, Kyd JM, Foxwell AR. Pseudomonas aeruginosa: the potential to immunise against infection. Exp Opin Biol Ther. 2005;5:967–982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

