Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods
- PMID: 23604020
- PMCID: PMC11113683
- DOI: 10.1007/s00018-013-1334-0
Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods
Erratum in
- Cell Mol Life Sci. 2013 Nov;70(21):4197. de Valdivia, Ernesto Gonzalez [corrected to Gonzalez de Valdivia, Ernesto]
Abstract
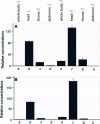
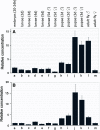
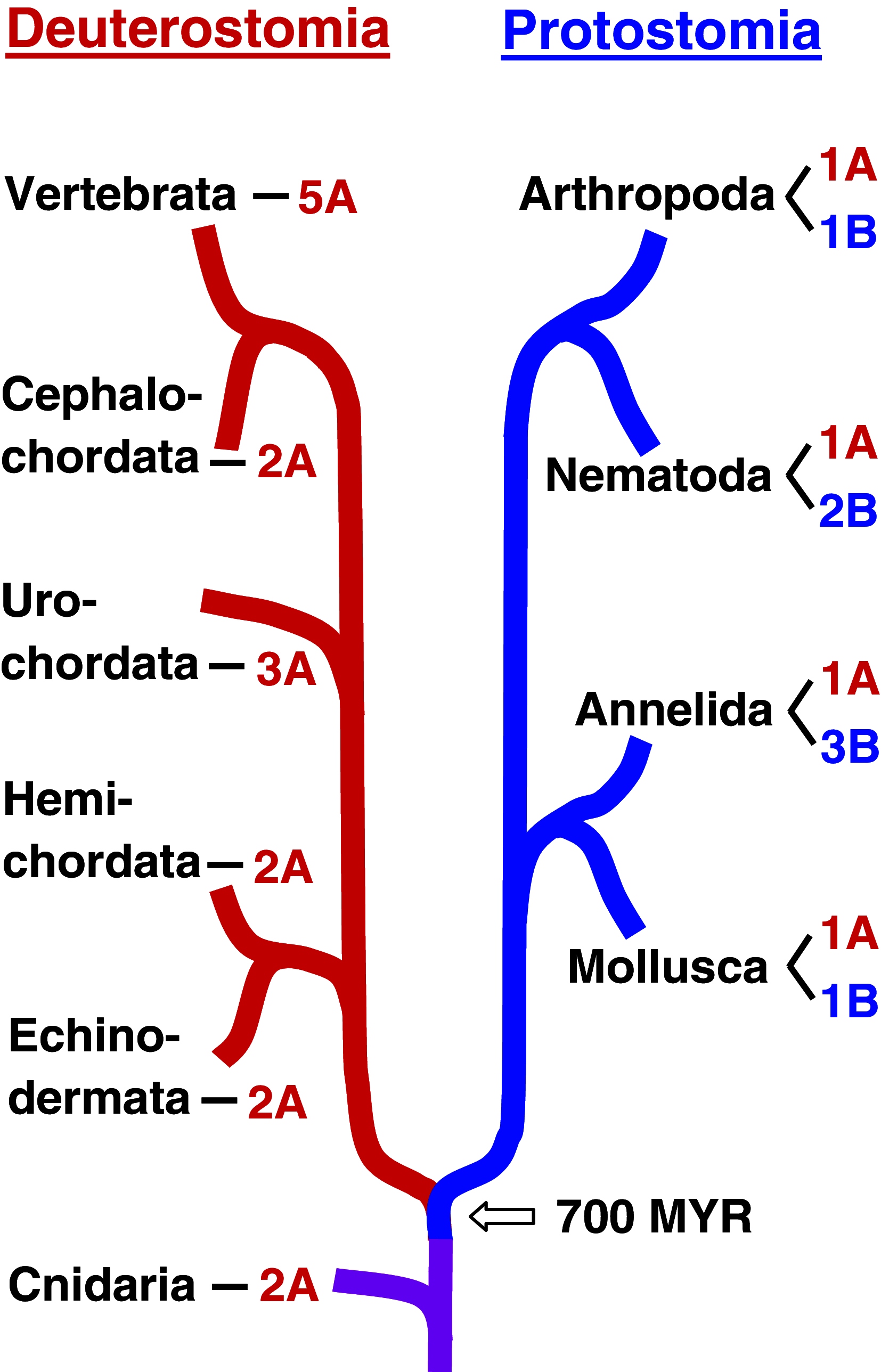
Muscarinic acetylcholine receptors (mAChRs) play a central role in the mammalian nervous system. These receptors are G protein-coupled receptors (GPCRs), which are activated by the agonists acetylcholine and muscarine, and blocked by a variety of antagonists. Mammals have five mAChRs (m1-m5). In this study, we cloned two structurally related GPCRs from the fruit fly Drosophila melanogaster, which, after expression in Chinese hamster ovary cells, proved to be muscarinic acetylcholine receptors. One mAChR (the A-type; encoded by gene CG4356) is activated by acetylcholine (EC50, 5 × 10(-8) M) and muscarine (EC50, 6 × 10(-8) M) and blocked by the classical mAChR antagonists atropine, scopolamine, and 3-quinuclidinyl-benzilate (QNB), while the other (the B-type; encoded by gene CG7918) is also activated by acetylcholine, but has a 1,000-fold lower sensitivity to muscarine, and is not blocked by the antagonists. A- and B-type mAChRs were also cloned and functionally characterized from the red flour beetle Tribolium castaneum. Recently, Haga et al. (Nature 2012, 482: 547-551) published the crystal structure of the human m2 mAChR, revealing 14 amino acid residues forming the binding pocket for QNB. These residues are identical between the human m2 and the D. melanogaster and T. castaneum A-type mAChRs, while many of them are different between the human m2 and the B-type receptors. Using bioinformatics, one orthologue of the A-type and one of the B-type mAChRs could also be found in all other arthropods with a sequenced genome. Protostomes, such as arthropods, and deuterostomes, such as mammals and other vertebrates, belong to two evolutionarily distinct lineages of animal evolution that split about 700 million years ago. We found that animals that originated before this split, such as cnidarians (Hydra), had two A-type mAChRs. From these data we propose a model for the evolution of mAChRs.
Figures



Similar articles
-
The A- and B-type muscarinic acetylcholine receptors from Drosophila melanogaster couple to different second messenger pathways.Biochem Biophys Res Commun. 2015 Jul 10;462(4):358-64. doi: 10.1016/j.bbrc.2015.04.141. Epub 2015 May 9. Biochem Biophys Res Commun. 2015. PMID: 25964087
-
A new family of insect muscarinic acetylcholine receptors.Insect Mol Biol. 2016 Aug;25(4):362-9. doi: 10.1111/imb.12229. Epub 2016 Mar 22. Insect Mol Biol. 2016. PMID: 27003873
-
Comparison of subcellular distribution and functions between exogenous and endogenous M1 muscarinic acetylcholine receptors.Life Sci. 2013 Jul 19;93(1):17-23. doi: 10.1016/j.lfs.2013.05.013. Epub 2013 May 30. Life Sci. 2013. PMID: 23727356
-
Molecular biology of muscarinic acetylcholine receptors.Crit Rev Neurobiol. 1996;10(1):69-99. doi: 10.1615/critrevneurobiol.v10.i1.40. Crit Rev Neurobiol. 1996. PMID: 8853955 Review.
-
A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum.Front Neuroendocrinol. 2008 Jan;29(1):142-65. doi: 10.1016/j.yfrne.2007.10.003. Epub 2007 Oct 24. Front Neuroendocrinol. 2008. PMID: 18054377 Review.
Cited by
-
Three-dimensional analysis of the heart function and effect cholinergic agonists in the cockroach Gromphadorhina portentosa.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Nov;206(6):857-870. doi: 10.1007/s00359-020-01443-5. Epub 2020 Sep 21. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 32955634 Free PMC article.
-
De novo assembly and characterization of central nervous system transcriptome reveals neurotransmitter signaling systems in the rice striped stem borer, Chilo suppressalis.BMC Genomics. 2015 Jul 15;16(1):525. doi: 10.1186/s12864-015-1742-7. BMC Genomics. 2015. PMID: 26173787 Free PMC article.
-
Cholinergic Agonists and Antagonists Have an Effect on the Metabolism of the Beetle Tenebrio Molitor.Molecules. 2018 Dec 20;24(1):17. doi: 10.3390/molecules24010017. Molecules. 2018. PMID: 30577556 Free PMC article.
-
Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning.Insects. 2022 Sep 5;13(9):806. doi: 10.3390/insects13090806. Insects. 2022. PMID: 36135508 Free PMC article.
-
Muscarinic Modulation of Antennal Lobe GABAergic Local Neurons Shapes Odor Coding and Behavior.Cell Rep. 2019 Dec 3;29(10):3253-3265.e4. doi: 10.1016/j.celrep.2019.10.125. Cell Rep. 2019. PMID: 31801087 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

