Emerging roles of astrocytes in neural circuit development
- PMID: 23595014
- PMCID: PMC4431630
- DOI: 10.1038/nrn3484
Emerging roles of astrocytes in neural circuit development
Erratum in
- Nat Rev Neurosci. 2013 Jun;14(6):451
Abstract
Astrocytes are now emerging as key participants in many aspects of brain development, function and disease. In particular, new evidence shows that astrocytes powerfully control the formation, maturation, function and elimination of synapses through various secreted and contact-mediated signals. Astrocytes are also increasingly being implicated in the pathophysiology of many psychiatric and neurological disorders that result from synaptic defects. A better understanding of how astrocytes regulate neural circuit development and function in the healthy and diseased brain might lead to the development of therapeutic agents to treat these diseases.
Conflict of interest statement
B.A.B. declares competing financial interests. See Web version for details. L.E.C. declares no competing financial interests.
Figures
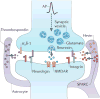
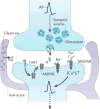

Similar articles
-
Astrocytic control of neural circuit formation: highlights on TGF-beta signaling.Neurochem Int. 2014 Dec;78:18-27. doi: 10.1016/j.neuint.2014.07.008. Epub 2014 Aug 11. Neurochem Int. 2014. PMID: 25125369 Review.
-
Neuron-astrocyte signaling is preserved in the aging brain.Glia. 2017 Apr;65(4):569-580. doi: 10.1002/glia.23112. Epub 2017 Jan 28. Glia. 2017. PMID: 28130845 Free PMC article.
-
Role for glia in synaptogenesis.Glia. 2004 Aug 15;47(3):209-216. doi: 10.1002/glia.20082. Glia. 2004. PMID: 15252809 Review.
-
Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity.J Neurochem. 2009 Feb;108(3):533-44. doi: 10.1111/j.1471-4159.2008.05830.x. J Neurochem. 2009. PMID: 19187090 Review.
-
Astrocyte regulation of synaptic behavior.Annu Rev Cell Dev Biol. 2014;30:439-63. doi: 10.1146/annurev-cellbio-100913-013053. Annu Rev Cell Dev Biol. 2014. PMID: 25288116 Review.
Cited by
-
Association of astrocytes with neurons and astrocytes derived from distinct progenitor domains in the subpallium.Sci Rep. 2015 Jul 20;5:12258. doi: 10.1038/srep12258. Sci Rep. 2015. PMID: 26193445 Free PMC article.
-
Grafted human-induced pluripotent stem cells-derived oligodendrocyte progenitor cells combined with human umbilical vein endothelial cells contribute to functional recovery following spinal cord injury.Stem Cell Res Ther. 2024 Feb 7;15(1):35. doi: 10.1186/s13287-024-03651-1. Stem Cell Res Ther. 2024. PMID: 38321505 Free PMC article.
-
Glia: guardians, gluttons, or guides for the maintenance of neuronal connectivity?Ann N Y Acad Sci. 2015 Sep;1351(1):1-10. doi: 10.1111/nyas.12711. Epub 2015 Mar 9. Ann N Y Acad Sci. 2015. PMID: 25752338 Free PMC article. Review.
-
Molecular Dissection of TDP-43 as a Leading Cause of ALS/FTLD.Int J Mol Sci. 2022 Oct 19;23(20):12508. doi: 10.3390/ijms232012508. Int J Mol Sci. 2022. PMID: 36293362 Free PMC article. Review.
-
Therapeutic advances in neural regeneration for Huntington's disease.Neural Regen Res. 2024 Sep 1;19(9):1991-1997. doi: 10.4103/1673-5374.390969. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227527 Free PMC article.
References
-
- Allen NJ, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. This study identifies a novel family of astrocyte-secreted proteins that recruit glutamate receptors to excitatory synapses to induce synapse maturation. - PMC - PubMed
-
- Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. This study identifies a novel family of astrocyte-secreted proteins that promotes the formation of excitatory synapses. - PubMed
-
- Hughes EG, Elmariah SB, Balice-Gordon RJ. Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol Cell Neurosci. 2010;43:136–145. This study demonstrates that in addition to releasing molecules that regulate excitatory synaptogenesis, astrocytes release different molecules to control inhibitory synaptogenesis. - PMC - PubMed
-
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. References 5 and 6 were the first studies to show that astrocytes can induce and control the number of excitatory synapses by secreting soluble signals. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

