iNOS: a potential therapeutic target for malignant glioma
- PMID: 23590833
- PMCID: PMC4266467
- DOI: 10.2174/1566524011313080002
iNOS: a potential therapeutic target for malignant glioma
Abstract
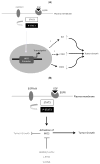
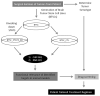
Glioblastoma is the most aggressive adult primary brain tumor. Although progress has been made in understanding the molecular mechanisms underlying these tumors, current treatments are ineffective. Recent studies have identified iNOS as a critical regulator of glial transformation downstream of EGFRvIII/STAT3 signaling, a key oncogenic pathway in glioblastoma. STAT3 directly binds the promoter of the iNOS gene and thereby stimulates its expression. Importantly, inhibition of iNOS by genetic and pharmacological approaches impedes glial cell proliferation, invasiveness, and tumor growth in vivo. iNOS expression is also elevated in a population of human brain tumor stem cells (BTSCs), and iNOS is required for BTSC proliferation and tumorigenesis. Together, these findings suggest that development of iNOS-targeted therapies may prove valuable in the treatment of glioblastoma. Here, we review our current understanding of iNOS signaling in the regulation of glioblastoma pathogenesis and the potential mechanisms by which iNOS inhibition might suppress the malignant behavior of these devastating tumors.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures
Similar articles
-
STAT3-iNOS Signaling Mediates EGFRvIII-Induced Glial Proliferation and Transformation.J Neurosci. 2012 Jun 6;32(23):7806-18. doi: 10.1523/JNEUROSCI.3243-11.2012. J Neurosci. 2012. PMID: 22674257 Free PMC article.
-
Transcriptional control of brain tumor stem cells by a carbohydrate binding protein.Cell Rep. 2021 Aug 31;36(9):109647. doi: 10.1016/j.celrep.2021.109647. Cell Rep. 2021. PMID: 34469737
-
Targeting iNOS As a Valuable Strategy for the Therapy of Glioma.ChemMedChem. 2020 Feb 17;15(4):339-344. doi: 10.1002/cmdc.201900580. Epub 2020 Jan 22. ChemMedChem. 2020. PMID: 31851765 Review.
-
On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells.Neuro Oncol. 2013 Feb;15(2):198-207. doi: 10.1093/neuonc/nos302. Epub 2012 Dec 21. Neuro Oncol. 2013. PMID: 23262510 Free PMC article.
-
Targeting ErbB receptors in high-grade glioma.Curr Pharm Des. 2011;17(23):2468-87. doi: 10.2174/138161211797249233. Curr Pharm Des. 2011. PMID: 21827413 Review.
Cited by
-
Gene expression profiles of NO- and HNO-donor treated breast cancer cells: insights into tumor response and resistance pathways.Nitric Oxide. 2014 Dec 1;43:17-28. doi: 10.1016/j.niox.2014.08.003. Epub 2014 Aug 19. Nitric Oxide. 2014. PMID: 25153034 Free PMC article. Review.
-
Involvement of NOS2 Activity on Human Glioma Cell Growth, Clonogenic Potential, and Neurosphere Generation.Int J Mol Sci. 2018 Sep 17;19(9):2801. doi: 10.3390/ijms19092801. Int J Mol Sci. 2018. PMID: 30227679 Free PMC article.
-
Modulation of the Anti-Tumor Efficacy of Photodynamic Therapy by Nitric Oxide.Cancers (Basel). 2016 Oct 20;8(10):96. doi: 10.3390/cancers8100096. Cancers (Basel). 2016. PMID: 27775600 Free PMC article. Review.
-
NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression.Oncotarget. 2017 Apr 11;8(15):25582-25598. doi: 10.18632/oncotarget.16106. Oncotarget. 2017. PMID: 28424427 Free PMC article.
-
Cochlear implantation impairs intracochlear microcirculation and counteracts iNOS induction in guinea pigs.Front Cell Neurosci. 2023 Jun 28;17:1189980. doi: 10.3389/fncel.2023.1189980. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37448696 Free PMC article.
References
-
- Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Robins HI, Chang S, Butowski N, Mehta M. Therapeutic advances for glioblastoma multiforme: current status and future prospects. Curr Oncol Reports. 2007;9:66–70. - PubMed
-
- Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

