Intra-osseous injection of donor mesenchymal stem cell (MSC) into the bone marrow in living donor kidney transplantation; a pilot study
- PMID: 23578110
- PMCID: PMC3630056
- DOI: 10.1186/1479-5876-11-96
Intra-osseous injection of donor mesenchymal stem cell (MSC) into the bone marrow in living donor kidney transplantation; a pilot study
Abstract
Background: Mesenchymal stem cells (MSCs) are multi-potent non-hematopoietic progenitor cells possessing an immune-regulatory function, with suppression of proliferation of activated lymphocytes. In this study, adult living donor kidney transplantation (LDKT) recipients were given MSCs derived from the donor bone marrow to evaluate the safety and the feasibility of immunological changes related to the intra-osseous injection of MSC into the bone marrow.
Methods: MSCs were derived from negative HLA cross-match donors. Donor bone marrow was harvested 5 weeks prior to KT. At the time of transplantation, 1 x 106 cell/kg of donor MSC was directly injected into the bone marrow of the recipient's right iliac bone. Patients' clinical outcomes, presence of mixed chimerism by short tandem repeat polymerase chain reaction, analysis of plasma FoxP3 mRNA and cytokine level, and mixed lymphocyte reaction (MLR) were performed.
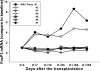
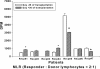
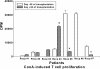
Results: Seven patients enrolled in this study and received donor MSC injections simultaneously with LDKT. The median age of recipients was 36 years (32 ~ 48). The number of HLA mismatches was 3 or less in 5 and more than 3 in 2. No local complications or adverse events such as hypersensitivity occurred during or after the injection of donor MSC. There was no graft failure, but the biopsy-proven acute rejections were observed in 3 recipients during the follow-up period controlled well with steroid pulse therapy (SPT). The last serum creatinine was a median of 1.23 mg/dL (0.83 ~ 2.07). Mixed chimerism was not detected in the peripheral blood of the recipients at 1 and 8 week of post-transplantation. Donor-specific lymphocyte or T cell proliferation and Treg priming responses were observed in some patients. Plasma level of IL-10, a known mediator of MSC-induced immune suppression, increased in the patients with Treg induction.
Conclusion: Donor MSC injection into the iliac bone at the time of KT was feasible and safe. A possible correlation was observed between the induction of inhibitory immune responses and the clinical outcome in the MSC-kidney transplanted patients. Further research will be performed to evaluate the efficacy of MSC injection for the induction of mixed chimerism and subsequent immune tolerance in KT.
Figures



Similar articles
-
A Large-Scale Bank of Organ Donor Bone Marrow and Matched Mesenchymal Stem Cells for Promoting Immunomodulation and Transplant Tolerance.Front Immunol. 2021 Feb 26;12:622604. doi: 10.3389/fimmu.2021.622604. eCollection 2021. Front Immunol. 2021. PMID: 33732244 Free PMC article. Review.
-
Allogeneic mesenchymal stem cells as induction therapy are safe and feasible in renal allografts: pilot results of a multicenter randomized controlled trial.J Transl Med. 2018 Mar 7;16(1):52. doi: 10.1186/s12967-018-1422-x. J Transl Med. 2018. PMID: 29514693 Free PMC article. Clinical Trial.
-
Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients.Transplantation. 1998 Apr 15;65(7):956-65. doi: 10.1097/00007890-199804150-00016. Transplantation. 1998. PMID: 9565101
-
Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study.Stem Cells Transl Med. 2013 Feb;2(2):107-11. doi: 10.5966/sctm.2012-0114. Epub 2013 Jan 24. Stem Cells Transl Med. 2013. PMID: 23349326 Free PMC article. Clinical Trial.
-
Hematopoietic cell-based and non-hematopoietic cell-based strategies for immune tolerance induction in living-donor renal transplantation: A systematic review.Transplant Rev (Orlando). 2023 Dec;37(4):100792. doi: 10.1016/j.trre.2023.100792. Epub 2023 Aug 19. Transplant Rev (Orlando). 2023. PMID: 37709652 Review.
Cited by
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2022 Dec;18(8):2872-2892. doi: 10.1007/s12015-022-10384-2. Epub 2022 May 19. Stem Cell Rev Rep. 2022. PMID: 35590083 Free PMC article.
-
Human dystrophin expressing chimeric (DEC) cell therapy ameliorates cardiac, respiratory, and skeletal muscle's function in Duchenne muscular dystrophy.Stem Cells Transl Med. 2021 Oct;10(10):1406-1418. doi: 10.1002/sctm.21-0054. Epub 2021 Jul 22. Stem Cells Transl Med. 2021. PMID: 34291884 Free PMC article.
-
Recent Progress in Cell Therapy in Solid Organ Transplantation.Int J Organ Transplant Med. 2017;8(3):125-131. Epub 2017 Aug 1. Int J Organ Transplant Med. 2017. PMID: 28924460 Free PMC article. Review.
-
Bone Fragment Co-transplantation Alongside Bone Marrow Aspirate Infusion Protects Kidney Transplant Recipients.Front Immunol. 2021 Feb 11;12:630710. doi: 10.3389/fimmu.2021.630710. eCollection 2021. Front Immunol. 2021. PMID: 33643315 Free PMC article.
-
Comparative Analysis of Biological Signatures between Freshly Preserved and Cryo-Preserved Bone Marrow Mesenchymal Stem Cells.Cells. 2023 Sep 26;12(19):2355. doi: 10.3390/cells12192355. Cells. 2023. PMID: 37830568 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

