A systematic screen of FDA-approved drugs for inhibitors of biological threat agents
- PMID: 23577127
- PMCID: PMC3618516
- DOI: 10.1371/journal.pone.0060579
A systematic screen of FDA-approved drugs for inhibitors of biological threat agents
Abstract
Background: The rapid development of effective medical countermeasures against potential biological threat agents is vital. Repurposing existing drugs that may have unanticipated activities as potential countermeasures is one way to meet this important goal, since currently approved drugs already have well-established safety and pharmacokinetic profiles in patients, as well as manufacturing and distribution networks. Therefore, approved drugs could rapidly be made available for a new indication in an emergency.
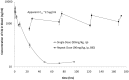
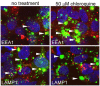
Methodology/principal findings: A large systematic effort to determine whether existing drugs can be used against high containment bacterial and viral pathogens is described. We assembled and screened 1012 FDA-approved drugs for off-label broad-spectrum efficacy against Bacillus anthracis; Francisella tularensis; Coxiella burnetii; and Ebola, Marburg, and Lassa fever viruses using in vitro cell culture assays. We found a variety of hits against two or more of these biological threat pathogens, which were validated in secondary assays. As expected, antibiotic compounds were highly active against bacterial agents, but we did not identify any non-antibiotic compounds with broad-spectrum antibacterial activity. Lomefloxacin and erythromycin were found to be the most potent compounds in vivo protecting mice against Bacillus anthracis challenge. While multiple virus-specific inhibitors were identified, the most noteworthy antiviral compound identified was chloroquine, which disrupted entry and replication of two or more viruses in vitro and protected mice against Ebola virus challenge in vivo.
Conclusions/significance: The feasibility of repurposing existing drugs to face novel threats is demonstrated and this represents the first effort to apply this approach to high containment bacteria and viruses.
Conflict of interest statement
Figures
Similar articles
-
High-throughput drug screening using the Ebola virus transcription- and replication-competent virus-like particle system.Antiviral Res. 2018 Oct;158:226-237. doi: 10.1016/j.antiviral.2018.08.013. Epub 2018 Aug 24. Antiviral Res. 2018. PMID: 30149038
-
Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection.J Virol. 2017 Oct 13;91(21):e01055-17. doi: 10.1128/JVI.01055-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814523 Free PMC article.
-
Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses.J Virol. 2019 Apr 3;93(8):e02185-18. doi: 10.1128/JVI.02185-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700611 Free PMC article.
-
Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs.Int J Biol Macromol. 2021 Mar 1;172:524-541. doi: 10.1016/j.ijbiomac.2021.01.076. Epub 2021 Jan 14. Int J Biol Macromol. 2021. PMID: 33454328 Free PMC article. Review.
-
Medical countermeasures for unwanted CBRN exposures: Part I chemical and biological threats with review of recent countermeasure patents.Expert Opin Ther Pat. 2016 Dec;26(12):1431-1447. doi: 10.1080/13543776.2017.1233178. Epub 2016 Sep 14. Expert Opin Ther Pat. 2016. PMID: 27599259 Review.
Cited by
-
The broad-spectrum antiviral recommendations for drug discovery against COVID-19.Drug Metab Rev. 2020 Aug;52(3):408-424. doi: 10.1080/03602532.2020.1770782. Epub 2020 Jun 17. Drug Metab Rev. 2020. PMID: 32546018 Free PMC article. Review.
-
Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS.Retrovirology. 2015 Jun 18;12:51. doi: 10.1186/s12977-015-0178-0. Retrovirology. 2015. PMID: 26084487 Free PMC article. Review.
-
Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus.Front Immunol. 2018 Aug 10;9:1803. doi: 10.3389/fimmu.2018.01803. eCollection 2018. Front Immunol. 2018. PMID: 30147687 Free PMC article. Review.
-
Identification of a clinical compound losmapimod that blocks Lassa virus entry.Antiviral Res. 2019 Jul;167:68-77. doi: 10.1016/j.antiviral.2019.03.014. Epub 2019 Apr 4. Antiviral Res. 2019. PMID: 30953674 Free PMC article.
-
Déjà vu: Stimulating open drug discovery for SARS-CoV-2.Drug Discov Today. 2020 May;25(5):928-941. doi: 10.1016/j.drudis.2020.03.019. Epub 2020 Apr 19. Drug Discov Today. 2020. PMID: 32320852 Free PMC article. Review.
References
-
- Center for Disease Control and Prevention (2013) Bioterrorism Agents/Diseases. Center for Disease Control and Prevention. Available: http://www.bt.cdc.gov/agent/agentlist-category.asp: Accessed 10 Feb 2013.
-
- Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, et al. (2002) Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA 287: 2236–2252. - PubMed
-
- Center for Disease Control and Prevention (2012) Anthrax. Available: http://emergency.cdc.gov/agent/anthrax/: Accessed 29 Feb 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

