Accelerated drug release and clearance of PEGylated epirubicin liposomes following repeated injections: a new challenge for sequential low-dose chemotherapy
- PMID: 23576868
- PMCID: PMC3616606
- DOI: 10.2147/IJN.S41701
Accelerated drug release and clearance of PEGylated epirubicin liposomes following repeated injections: a new challenge for sequential low-dose chemotherapy
Abstract
Background: Sequential low-dose chemotherapy has received great attention for its unique advantages in attenuating multidrug resistance of tumor cells. Nevertheless, it runs the risk of producing new problems associated with the accelerated blood clearance phenomenon, especially with multiple injections of PEGylated liposomes.
Methods: Liposomes were labeled with fluorescent phospholipids of 1,2-dipalmitoyl-snglycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) and epirubicin (EPI). The pharmacokinetics profile and biodistribution of the drug and liposome carrier following multiple injections were determined. Meanwhile, the antitumor effect of sequential low-dose chemotherapy was tested. To clarify this unexpected phenomenon, the production of polyethylene glycol (PEG)-specific immunoglobulin M (IgM), drug release, and residual complement activity experiments were conducted in serum.
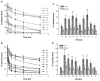
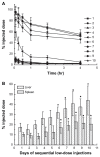
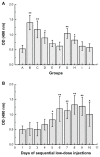
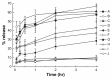
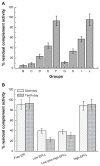
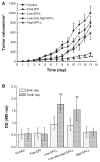
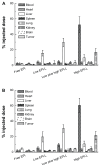
Results: The first or sequential injections of PEGylated liposomes within a certain dose range induced the rapid clearance of subsequently injected PEGylated liposomal EPI. Of note, the clearance of EPI was two- to three-fold faster than the liposome itself, and a large amount of EPI was released from liposomes in the first 30 minutes in a complement-activation, direct-dependent manner. The therapeutic efficacy of liposomal EPI following 10 days of sequential injections in S180 tumor-bearing mice of 0.75 mg EPI/kg body weight was almost completely abolished between the sixth and tenth day of the sequential injections, even although the subsequently injected doses were doubled. The level of PEG-specific IgM in the blood increased rapidly, with a larger amount of complement being activated while the concentration of EPI in blood and tumor tissue was significantly reduced.
Conclusion: Our investigation implied that the accelerated blood clearance phenomenon and its accompanying rapid leakage and clearance of drug following sequential low-dose injections may reverse the unique pharmacokinetic-toxicity profile of liposomes which deserved our attention. Therefore, a more reasonable treatment regime should be selected to lessen or even eliminate this phenomenon.
Keywords: PEGylated liposomes; accelerated blood clearance (ABC) phenomenon; complement; epirubicin; sequential low-dose injections.
Figures
Similar articles
-
Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection.J Control Release. 2006 Oct 27;115(3):251-8. doi: 10.1016/j.jconrel.2006.08.017. Epub 2006 Sep 3. J Control Release. 2006. PMID: 17045355
-
Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration.Int J Pharm. 2013 Nov 1;456(1):235-42. doi: 10.1016/j.ijpharm.2013.07.059. Epub 2013 Aug 5. Int J Pharm. 2013. PMID: 23928149
-
Accelerated blood clearance of pegylated liposomal topotecan: influence of polyethylene glycol grafting density and animal species.J Pharm Sci. 2012 Oct;101(10):3864-76. doi: 10.1002/jps.23254. Epub 2012 Jul 6. J Pharm Sci. 2012. PMID: 22777607
-
Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes.Int J Pharm. 2008 Apr 16;354(1-2):56-62. doi: 10.1016/j.ijpharm.2007.11.005. Epub 2007 Nov 9. Int J Pharm. 2008. PMID: 18083313 Review.
-
[Accelerated blood clearance (ABC) phenomenon induced by administration of PEGylated liposome].Yakugaku Zasshi. 2008 Feb;128(2):233-43. doi: 10.1248/yakushi.128.233. Yakugaku Zasshi. 2008. PMID: 18239370 Review. Japanese.
Cited by
-
The effect of monosialylganglioside mix modifying the PEGylated liposomal epirubicin on the accelerated blood clearance phenomenon.Asian J Pharm Sci. 2017 Mar;12(2):134-142. doi: 10.1016/j.ajps.2016.06.005. Epub 2016 Aug 4. Asian J Pharm Sci. 2017. PMID: 32104322 Free PMC article.
-
Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes.Int J Mol Sci. 2017 Jun 10;18(6):1249. doi: 10.3390/ijms18061249. Int J Mol Sci. 2017. PMID: 28604595 Free PMC article. Review.
-
Anti-PEG immunity: emergence, characteristics, and unaddressed questions.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 Sep-Oct;7(5):655-77. doi: 10.1002/wnan.1339. Epub 2015 Feb 23. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25707913 Free PMC article. Review.
-
Main trends of immune effects triggered by nanomedicines in preclinical studies.Int J Nanomedicine. 2018 Sep 17;13:5419-5431. doi: 10.2147/IJN.S168808. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30271138 Free PMC article. Review.
-
Anchoring Property of a Novel Hydrophilic Lipopolymer, HDAS-SHP, Post-Inserted in Preformed Liposomes.Nanomaterials (Basel). 2019 Aug 21;9(9):1185. doi: 10.3390/nano9091185. Nanomaterials (Basel). 2019. PMID: 31438526 Free PMC article.
References
-
- Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–321. - PubMed
-
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. - PubMed
-
- Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29–36. - PubMed
-
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. - PubMed
-
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

