Hydroxyproline-free single composition ABC collagen heterotrimer
- PMID: 23574286
- PMCID: PMC3663077
- DOI: 10.1021/ja402187t
Hydroxyproline-free single composition ABC collagen heterotrimer
Abstract
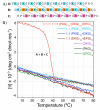
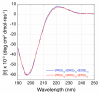
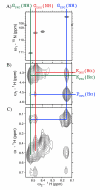
Hydroxyproline plays a major role in stabilizing collagenous domains in eukaryotic organisms. Lack of this modification is associated with significant lowering in the thermal stability of the collagen triple helix and may also affect fibrillogenesis and folding of the peptide chains. In contrast, even though bacterial collagens lack hydroxyproline, their thermal stability is comparable to that of fibrillar collagen. This has been attributed to the high frequency of charged amino acids found in bacterial collagen. Here we report a thermally stable hydroxyproline-free ABC heterotrimeric collagen mimetic system composed of decapositive and decanegative peptides and a zwitterionic peptide. None of the peptides contain hydroxyproline, and furthermore the zwitterionic peptide does not even contain proline. The heterotrimer is electrostatically stabilized via multiple interpeptide lysine-aspartate and lysine-glutamate salt bridges and maintains good thermal stability with a melting temperature of 37 °C. The ternary peptide mixture also populates a single composition ABC heterotrimer as confirmed by circular dichroism (CD) and nuclear magnetic resonance (NMR) spectroscopy. This system illustrates the power of axial salt bridges to direct and stabilize the self-assembly of a triple helix and may be useful in analogous designs in expression systems where the incorporation of hydroxyproline is challenging.
Figures

Similar articles
-
Supramolecular assembly of electrostatically stabilized, hydroxyproline-lacking collagen-mimetic peptides.Biomacromolecules. 2009 Sep 14;10(9):2626-31. doi: 10.1021/bm900551c. Biomacromolecules. 2009. PMID: 19681603 Free PMC article.
-
Surprisingly high stability of collagen ABC heterotrimer: evaluation of side chain charge pairs.J Am Chem Soc. 2007 Dec 5;129(48):15034-41. doi: 10.1021/ja075854z. Epub 2007 Nov 8. J Am Chem Soc. 2007. PMID: 17988128
-
Positive and negative design leads to compositional control in AAB collagen heterotrimers.J Am Chem Soc. 2011 Apr 13;133(14):5432-43. doi: 10.1021/ja111239r. Epub 2011 Mar 23. J Am Chem Soc. 2011. PMID: 21428435
-
Insights on the conformational stability of collagen.Nat Prod Rep. 2002 Feb;19(1):49-59. doi: 10.1039/a903001h. Nat Prod Rep. 2002. PMID: 11902439 Review.
-
The crucial role of trimerization domains in collagen folding.Int J Biochem Cell Biol. 2012 Jan;44(1):21-32. doi: 10.1016/j.biocel.2011.09.009. Epub 2011 Oct 5. Int J Biochem Cell Biol. 2012. PMID: 22001560 Review.
Cited by
-
Impact of collagen-like peptide (CLP) heterotrimeric triple helix design on helical thermal stability and hierarchical assembly: a coarse-grained molecular dynamics simulation study.Soft Matter. 2022 Apr 20;18(16):3177-3192. doi: 10.1039/d2sm00087c. Soft Matter. 2022. PMID: 35380571 Free PMC article.
-
Hollow Octadecameric Self-Assembly of Collagen-like Peptides.J Am Chem Soc. 2023 Mar 8;145(9):5285-5296. doi: 10.1021/jacs.2c12931. Epub 2023 Feb 22. J Am Chem Soc. 2023. PMID: 36812303 Free PMC article.
-
Selective covalent capture of collagen triple helices with a minimal protecting group strategy.Chem Sci. 2022 Feb 17;13(9):2789-2796. doi: 10.1039/d1sc06361h. eCollection 2022 Mar 2. Chem Sci. 2022. PMID: 35356674 Free PMC article.
-
Engineered recombinant bacterial collagen as an alternative collagen-based biomaterial for tissue engineering.Front Chem. 2014 Jun 23;2:40. doi: 10.3389/fchem.2014.00040. eCollection 2014. Front Chem. 2014. PMID: 25003103 Free PMC article. No abstract available.
-
Histological and biochemical study of the superficial abdominal fascia and its implication in obesity.Anat Cell Biol. 2016 Sep;49(3):184-188. doi: 10.5115/acb.2016.49.3.184. Epub 2016 Sep 29. Anat Cell Biol. 2016. PMID: 27722011 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

