Unintentional transfer of vaccinia virus associated with smallpox vaccines: ACAM2000(®) compared with Dryvax(®)
- PMID: 23571177
- PMCID: PMC9491130
- DOI: 10.4161/hv.24319
Unintentional transfer of vaccinia virus associated with smallpox vaccines: ACAM2000(®) compared with Dryvax(®)
Abstract
Background: Routine vaccination against smallpox (variola) ceased in the US in 1976. However, in 2002 limited coverage for military personnel and some healthcare workers was reinstituted. In March 2008, ACAM2000® replaced Dryvax® as the vaccine used in the United States against smallpox. Unintentional transfer of vaccinia virus from a vaccination site by autoinoculation or contact transmission, can have significant public health implications. We summarize unintentional virus transfer AEs associated with ACAM2000® since March 2008 and compare with Dryvax®.
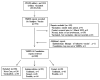
Results: We identified 309 reports for ACAM2000® with skin or ocular involvement, of which 93 were autoinoculation cases and 20 were contact transmission cases. The rate for reported cases of autoinoculation was 20.6 per 100,000 vaccinations and for contact transmission was 4.4 per 100,000 vaccinations. Eighteen contact transmission cases could be attributed to contact during a sporting activity (45%) or intimate contact (45%). Of the 113 unintentional transfer cases, 6 met the case definition for ocular vaccinia. The most common locations for all autoinoculation and contact cases were arm/elbow/shoulder (35/113; 31%) and face (24/113; 21%). Methods We reviewed 753 reports associated with smallpox in the Vaccine Adverse Event Reporting System and CDC Poxvirus consultation log, reported from March 2008 to August 2010. Reports were classified into categories based upon standard case definitions.
Conclusion: Overall, unintentional transfer events for ACAM2000® and Dryvax® are similar. We recommend continued efforts to prevent transfer events and continuing education for healthcare providers focused on recognition of vaccinia lesions, proper sample collection, and laboratory testing to confirm diagnosis.
Keywords: Orthopoxvirus; adverse events; autoinoculation; contact vaccinia; smallpox vaccine; vaccinia virus.
Figures


Similar articles
-
Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC).MMWR Recomm Rep. 2003 Apr 4;52(RR-7):1-16. MMWR Recomm Rep. 2003. PMID: 12710832
-
Smallpox vaccination and adverse reactions. Guidance for clinicians.MMWR Recomm Rep. 2003 Feb 21;52(RR-4):1-28. MMWR Recomm Rep. 2003. PMID: 12617510
-
Contact transmission of vaccinia virus from smallpox vaccinees in the United States, 2003-2011.Vaccine. 2012 Feb 1;30(6):985-8. doi: 10.1016/j.vaccine.2011.12.049. Epub 2011 Dec 20. Vaccine. 2012. PMID: 22192851
-
ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile.Drug Des Devel Ther. 2010 May 25;4:71-9. doi: 10.2147/dddt.s3687. Drug Des Devel Ther. 2010. PMID: 20531961 Free PMC article. Review.
-
ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine.Expert Opin Investig Drugs. 2008 Apr;17(4):555-64. doi: 10.1517/13543784.17.4.555. Expert Opin Investig Drugs. 2008. PMID: 18363519 Free PMC article. Review.
Cited by
-
Monkeypox virus: An emerging epidemic.Microb Pathog. 2022 Dec;173(Pt A):105794. doi: 10.1016/j.micpath.2022.105794. Epub 2022 Sep 28. Microb Pathog. 2022. PMID: 36179973 Free PMC article. Review.
-
A Review of Monkeypox Ocular Manifestations and Complications: Insights for the 2022 Outbreak.Ophthalmol Ther. 2023 Feb;12(1):55-69. doi: 10.1007/s40123-022-00626-4. Epub 2022 Dec 13. Ophthalmol Ther. 2023. PMID: 36512187 Free PMC article. Review.
-
Vaccination of Adults With Cancer: ASCO Guideline.J Clin Oncol. 2024 May 10;42(14):1699-1721. doi: 10.1200/JCO.24.00032. Epub 2024 Mar 18. J Clin Oncol. 2024. PMID: 38498792
-
Tracing the journey of poxviruses: insights from history.Arch Virol. 2024 Jan 27;169(2):37. doi: 10.1007/s00705-024-05971-2. Arch Virol. 2024. PMID: 38280957 Review.
-
Safety and Efficacy of Post-Eradication Smallpox Vaccine as an Mpox Vaccine: A Systematic Review with Meta-Analysis.Int J Environ Res Public Health. 2023 Feb 8;20(4):2963. doi: 10.3390/ijerph20042963. Int J Environ Res Public Health. 2023. PMID: 36833653 Free PMC article. Review.
References
-
- Greenberg M. . Complications of vaccination against smallpox. Am J Dis Child 1948; 76:492 - 502; PMID: 18108416 - PubMed
-
- Leake JP. . Question and answers on smallpox vaccination. Public Health Rep 1927; 42:221 - 38; http://dx.doi.org/10.2307/4578155; PMID: 19315071 - DOI - PubMed
-
- Neff JM, Lane JM, Pert JH, Moore R, Millar JD, Henderson DA. . Complications of smallpox vaccination. I. National survey in the United States, 1963. N Engl J Med 1967; 276:125 - 32; http://dx.doi.org/10.1056/NEJM196701192760301; PMID: 4381041 - DOI - PubMed
-
- Neff JM, Levine RH, Lane JM, Ager EA, Moore H, Rosenstein BJ, et al. . . Complications of smallpox vaccination United States 1963. II. Results obtained by four statewide surveys. Pediatrics 1967; 39:916 - 23; PMID: 4381735 - PubMed
-
- Lane JM, Ruben FL, Neff JM, Millar JD. . Complications of smallpox vaccination, 1968. N Engl J Med 1969; 281:1201 - 8; http://dx.doi.org/10.1056/NEJM196911272812201; PMID: 4186802 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
