MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response
- PMID: 23569328
- PMCID: PMC3646497
- DOI: 10.1084/jem.20121437
MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response
Abstract
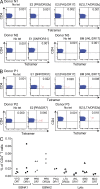
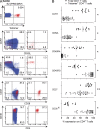
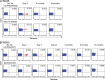
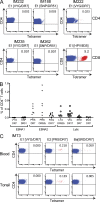
Virus-specific CD4(+) T cells are key orchestrators of host responses to viral infection yet, compared with their CD8(+) T cell counterparts, remain poorly characterized at the single cell level. Here we use nine MHC II-epitope peptide tetramers to visualize human CD4(+) T cell responses to Epstein-Barr virus (EBV), the causative agent of infectious mononucleosis (IM), a disease associated with large virus-specific CD8(+) T cell responses. We find that, while not approaching virus-specific CD8(+) T cell expansions in magnitude, activated CD4(+) T cells specific for epitopes in the latent antigen EBNA2 and four lytic cycle antigens are detected at high frequencies in acute IM blood. They then fall rapidly to values typical of life-long virus carriage where most tetramer-positive cells display conventional memory markers but some, unexpectedly, revert to a naive-like phenotype. In contrast CD4(+) T cell responses to EBNA1 epitopes are greatly delayed in IM patients, in line with the well-known but hitherto unexplained delay in EBNA1 IgG antibody responses. We present evidence from an in vitro system that may explain these unusual kinetics. Unlike other EBNAs and lytic cycle proteins, EBNA1 is not naturally released from EBV-infected cells as a source of antigen for CD4(+) T cell priming.
Figures




Similar articles
-
T cell detection of a B-cell tropic virus infection: newly-synthesised versus mature viral proteins as antigen sources for CD4 and CD8 epitope display.PLoS Pathog. 2009 Dec;5(12):e1000699. doi: 10.1371/journal.ppat.1000699. Epub 2009 Dec 18. PLoS Pathog. 2009. PMID: 20019813 Free PMC article.
-
Specificity of T cells in synovial fluid: high frequencies of CD8(+) T cells that are specific for certain viral epitopes.Arthritis Res. 2000;2(2):154-64. doi: 10.1186/ar80. Epub 2000 Feb 7. Arthritis Res. 2000. PMID: 11062606 Free PMC article.
-
Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma.J Virol. 2004 Jan;78(2):768-78. doi: 10.1128/jvi.78.2.768-778.2004. J Virol. 2004. PMID: 14694109 Free PMC article.
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy.PLoS One. 2007 Jul 4;2(7):e583. doi: 10.1371/journal.pone.0000583. PLoS One. 2007. PMID: 17611619 Free PMC article. Review.
Cited by
-
Mimicking the brain: Epstein-Barr virus and foreign agents as drivers of neuroimmune attack in multiple sclerosis.Front Immunol. 2023 Nov 3;14:1304281. doi: 10.3389/fimmu.2023.1304281. eCollection 2023. Front Immunol. 2023. PMID: 38022632 Free PMC article. Review.
-
Cytomegalovirus Infection Leads to Development of High Frequencies of Cytotoxic Virus-Specific CD4+ T Cells Targeted to Vascular Endothelium.PLoS Pathog. 2016 Sep 8;12(9):e1005832. doi: 10.1371/journal.ppat.1005832. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27606804 Free PMC article.
-
Immunology of THymectomy And childhood CArdiac transplant (ITHACA): protocol for a UK-wide prospective observational cohort study to identify immunological risk factors of post-transplant lymphoproliferative disease (PTLD) in thymectomised children.BMJ Open. 2023 Oct 21;13(10):e079582. doi: 10.1136/bmjopen-2023-079582. BMJ Open. 2023. PMID: 37865406 Free PMC article.
-
MHC class II tetramers made from isolated recombinant α and β chains refolded with affinity-tagged peptides.PLoS One. 2013 Sep 2;8(9):e73648. doi: 10.1371/journal.pone.0073648. eCollection 2013. PLoS One. 2013. PMID: 24023895 Free PMC article.
-
Engaging Natural Killer T Cells as 'Universal Helpers' for Vaccination.Drugs. 2017 Jan;77(1):1-15. doi: 10.1007/s40265-016-0675-z. Drugs. 2017. PMID: 28005229 Review.
References
-
- Amyes E., McMichael A.J., Callan M.F. 2005. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J. Immunol. 175:5765–5773 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

