Understanding heterogeneity in response to antidiabetes treatment: a post hoc analysis using SIDES, a subgroup identification algorithm
- PMID: 23567001
- PMCID: PMC3737644
- DOI: 10.1177/193229681300700219
Understanding heterogeneity in response to antidiabetes treatment: a post hoc analysis using SIDES, a subgroup identification algorithm
Abstract
Background: Treatment response in patients with type 2 diabetes mellitus (T2DM) varies because of different genotypic and phenotypic characteristics. Results of clinical trials are based largely on aggregated estimates of treatment effect rather than individualized outcomes. This research assessed heterogeneity and differential treatment response using the subgroup identification based on differential effect search (SIDES) algorithm with data from a large multinational study.
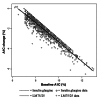
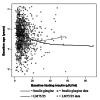
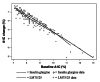
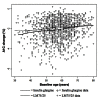
Methods: This was a retrospective analysis of the DURABLE trial, a randomized, open-label, two-arm, parallel study. The trial enrolled 2091 insulin-naïve T2DM patients aged 30 to 80 years. Patients received twice-daily insulin lispro mix 75/25 (LM75/25) or once-daily insulin glargine (insulin glargine). The SIDES methodology was used to find subgroups where the treatment effect was substantially different from what was observed in the full population of the clinical trial. A subgroup identification tool implementing the SIDES algorithm was used to examine data for 1092 patients (584 LM75/25 and 508 insulin glargine), achieving at-goal 12- or 24-week glycated hemoglobin A1c (A1C) (≤7.0%).
Results: The overall at-goal population treatment difference (A1C reduction) was not clinically meaningful, but a clinically meaningful difference was observed (A1C reduction 2.31% ± 0.06% LM75/25 versus 2.01% ± 0.07% insulin glargine; p = .001) in patients with a baseline fasting insulin level >11.4 μIU/ml and age ≤56 years.
Conclusion: The observation that younger patients with higher levels of fasting insulin may benefit from a regimen that includes short-acting insulin targeting postprandial glycemia helps explain the heterogeneity in response and warrants further investigation.
© 2013 Diabetes Technology Society.
Figures
Similar articles
-
Durability of glycemic control with insulin lispro mix 75/25 versus insulin glargine for older patients with type 2 diabetes.Aging Clin Exp Res. 2014 Apr;26(2):115-21. doi: 10.1007/s40520-013-0140-8. Epub 2013 Oct 3. Aging Clin Exp Res. 2014. PMID: 24092662 Free PMC article. Clinical Trial.
-
The DURAbility of Basal versus Lispro mix 75/25 insulin Efficacy (DURABLE) trial: comparing the durability of lispro mix 75/25 and glargine.Diabetes Care. 2011 Feb;34(2):249-55. doi: 10.2337/dc10-1701. Diabetes Care. 2011. PMID: 21270182 Free PMC article. Clinical Trial.
-
DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes.Diabetes Care. 2009 Jun;32(6):1007-13. doi: 10.2337/dc08-2117. Epub 2009 Mar 31. Diabetes Care. 2009. PMID: 19336625 Free PMC article. Clinical Trial.
-
Insulin glargine: a systematic review of a long-acting insulin analogue.Clin Ther. 2003 Jun;25(6):1541-77, discussion 1539-40. doi: 10.1016/s0149-2918(03)80156-x. Clin Ther. 2003. PMID: 12860485 Review.
-
Data-Driven Subgroup Identification in Confirmatory Clinical Trials.Ther Innov Regul Sci. 2022 Jan;56(1):65-75. doi: 10.1007/s43441-021-00329-1. Epub 2021 Jul 29. Ther Innov Regul Sci. 2022. PMID: 34327673 Review.
Cited by
-
The Identification of Diabetes Mellitus Subtypes Applying Cluster Analysis Techniques: A Systematic Review.Int J Environ Res Public Health. 2020 Dec 18;17(24):9523. doi: 10.3390/ijerph17249523. Int J Environ Res Public Health. 2020. PMID: 33353219 Free PMC article.
-
Predicting short- and long-term glycated haemoglobin response after insulin initiation in patients with type 2 diabetes mellitus using machine-learning algorithms.Diabetes Obes Metab. 2019 Dec;21(12):2704-2711. doi: 10.1111/dom.13860. Epub 2019 Sep 30. Diabetes Obes Metab. 2019. PMID: 31453664 Free PMC article.
-
Basal insulin combined incretin mimetic therapy with glucagon-like protein 1 receptor agonists as an upcoming option in the treatment of type 2 diabetes: a practical guide to decision making.Ther Adv Endocrinol Metab. 2014 Oct;5(5):95-123. doi: 10.1177/2042018814556099. Ther Adv Endocrinol Metab. 2014. PMID: 25419451 Free PMC article. Review.
-
The Combined Effect of Neuromuscular Electrical Stimulation and Insulin Therapy on Glycated Hemoglobin Concentrations, Lipid Profiles and Hemodynamic Parameters in Patients with Type-2-Diabetes and Hemiplegia Related to Ischemic Stroke: A Pilot Study.Int J Environ Res Public Health. 2021 Mar 26;18(7):3433. doi: 10.3390/ijerph18073433. Int J Environ Res Public Health. 2021. PMID: 33810235 Free PMC article.
-
Constructing treatment decision rules based on scalar and functional predictors when moderators of treatment effect are unknown.J R Stat Soc Ser C Appl Stat. 2018 Nov;67(5):1331-1356. doi: 10.1111/rssc.12278. Epub 2018 Apr 16. J R Stat Soc Ser C Appl Stat. 2018. PMID: 30546161 Free PMC article.
References
-
- World Health Organization. Diabetes. Fact sheet. No. 312. http://www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed April 12, 2012.
-
- Centers for Disease Control and Prevention. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.
-
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. - PubMed
-
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association; European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. - PMC - PubMed
-
- Shomali ME. Practical applications of therapy with a glucagon-like peptide-1 receptor agonist. J Fam Pract. 2009;58(9 Suppl Treating):S35–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

