Dictyostelium discoideum SecG interprets cAMP-mediated chemotactic signals to influence actin organization
- PMID: 23564751
- PMCID: PMC3693759
- DOI: 10.1002/cm.21107
Dictyostelium discoideum SecG interprets cAMP-mediated chemotactic signals to influence actin organization
Abstract
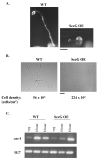
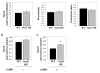
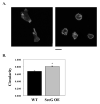
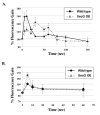
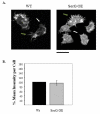
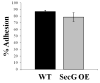
Tight control of actin cytoskeletal dynamics is essential for proper cell function and survival. Arf nucleotide binding-site opener (ARNO), a mammalian guanine nucleotide exchange factor for Arf, has been implicated in actin cytoskeletal regulation but its exact role is still unknown. To explore the role of ARNO in this regulation as well as in actin-mediated processes, the Dictyostelium discoideum homolog, SecG, was examined. SecG peaks during aggregation and mound formation. The overexpression of SecG arrests development at the mound stage. SecG overexpressing (SecG OE) cells fail to stream during aggregation. Although carA is expressed, SecG OE cells do not chemotax toward cAMP, indicating SecG is involved in the cellular response to cAMP. This chemotactic defect is specific to cAMP-directed chemotaxis, as SecG OE cells chemotax to folate without impairment and exhibit normal cell motility. The chemotactic defects of the SecG mutants may be due to an impaired cAMP response as evidenced by altered cell polarity and F-actin polymerization after cAMP stimulation. Cells overexpressing SecG have increased filopodia compared to wild type cells, implying that excess SecG causes abnormal organization of F-actin. The general function of the cytoskeleton, however, is not disrupted as the SecG OE cells exhibit proper cell-substrate adhesion. Taken together, the results suggest proper SecG levels are needed for appropriate response to cAMP signaling in order to coordinate F-actin organization during development.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

Similar articles
-
PakD, a putative p21-activated protein kinase in Dictyostelium discoideum, regulates actin.Eukaryot Cell. 2014 Jan;13(1):119-26. doi: 10.1128/EC.00216-13. Epub 2013 Nov 15. Eukaryot Cell. 2014. PMID: 24243792 Free PMC article.
-
The cyclase-associated protein CAP as regulator of cell polarity and cAMP signaling in Dictyostelium.Mol Biol Cell. 2004 Feb;15(2):934-45. doi: 10.1091/mbc.e03-05-0269. Epub 2003 Oct 31. Mol Biol Cell. 2004. PMID: 14595119 Free PMC article.
-
Transduction of the chemotactic signal to the actin cytoskeleton of Dictyostelium discoideum.Dev Biol. 1989 Dec;136(2):517-25. doi: 10.1016/0012-1606(89)90277-7. Dev Biol. 1989. PMID: 2511051
-
Cell migration: regulation of cytoskeleton by Rap1 in Dictyostelium discoideum.J Microbiol. 2012 Aug;50(4):555-61. doi: 10.1007/s12275-012-2246-7. Epub 2012 Aug 25. J Microbiol. 2012. PMID: 22923101 Review.
-
Ion Signaling in Cell Motility and Development in Dictyostelium discoideum.Biomolecules. 2024 Jul 10;14(7):830. doi: 10.3390/biom14070830. Biomolecules. 2024. PMID: 39062545 Free PMC article. Review.
Cited by
-
The C Isoform of Dictyostelium Tetraspanins Localizes to the Contractile Vacuole and Contributes to Resistance against Osmotic Stress.PLoS One. 2016 Sep 6;11(9):e0162065. doi: 10.1371/journal.pone.0162065. eCollection 2016. PLoS One. 2016. PMID: 27597994 Free PMC article.
-
ATP levels influence cell movement during the mound phase in Dictyostelium discoideum as revealed by ATP visualization and simulation.FEBS Open Bio. 2022 Nov;12(11):2042-2056. doi: 10.1002/2211-5463.13480. Epub 2022 Sep 23. FEBS Open Bio. 2022. PMID: 36054629 Free PMC article.
References
-
- Araki T, Abe T, Williams JG, Maeda Y. Symmetry breaking in Dictyostelium morphogenesis: Evidence that a combination of cell cycle stage and positional information dictates cell fate. Dev. Biol. 1997;192:645–648. - PubMed
-
- Blaauw M, Linskens MH, van Haastert PJ. Efficient control of gene expression by a tetracycline-dependent transactivator in single Dictyostelium discoideum cells. Gene. 2000;252(1-2):71–82. - PubMed
-
- Booth EO, Van Driessche N, Zhuchenko O, Kuspa A, Shaulsky G. Microarray phenotyping in Dictyostelium reveals a regulon of chemotaxis genes. Bioinformatics. 2005;21(24):4371–7. - PubMed
-
- Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384(6608):481–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

