Reassessing the detection of B-virus-specific serum antibodies
- PMID: 23561886
- PMCID: PMC3527757
Reassessing the detection of B-virus-specific serum antibodies
Abstract
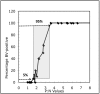
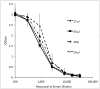
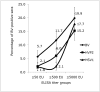
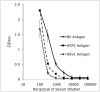
B virus, a natural pathogen of macaques, can cause a fatal zoonotic disease in humans. Serologic screening of macaques by titration ELISA (tELISA, screening test) and by Western blot analysis (WBA, confirmatory test) is one of the principle measures to prevent human infection. Here we slightly modified these 2 tests and reevaluated their correlation. We developed a high-throughput tELISA and used it to screen 278 sera simultaneously against the homologous BV antigen and the heterologous antigens of Papiine herpesvirus 2 and Human herpesvirus 1. More sera (35.6%) were positive by the BV-ELISA than by the HVP2-ELISA (21.6%) or HSV1-ELISA (19.8%). The superiority of the homologous tELISA over the heterologous tELISA was prominent in low-titer sera. WBA confirmed only 21% of the tELISA-positive sera with low or intermediate antibody titers. These sera might have contained antibodies to conformational epitopes that could not be detected by WBA, in which denatured antigens are used, but that could be detected by tELISA, which detects both linear and conformational epitopes. WBA confirmed 82% of the tELISA high-titer sera. However, WBA defined the remaining 18% of sera, which were negative by tELISA, as nonnegative. This finding can be attributed to the difficulties encountered with the subjective interpretation of results by WBA. Together, the current results indicate the inadequacy of WBA as a confirmatory assay for sera with low antibody titers.
Figures
Similar articles
-
An automated ELISA using recombinant antigens for serologic diagnosis of B virus infections in macaques.Comp Med. 2012 Dec;62(6):527-34. Comp Med. 2012. PMID: 23561887 Free PMC article.
-
Validation of an enzyme-linked immunosorbent assay kit using herpesvirus papio 2 (HVP2) antigen for detection of herpesvirus simiae (B virus) infection in rhesus monkeys.Comp Med. 2005 Jun;55(3):244-8. Comp Med. 2005. PMID: 16089172
-
Herpesvirus papio 2: alternative antigen for use in monkey B virus diagnostic assays.Lab Anim Sci. 1999 Dec;49(6):605-16. Lab Anim Sci. 1999. PMID: 10638495
-
Use of herpesvirus papio 2 as an alternative antigen in immunoblotting assay for B virus diagnosis.J Vet Med Sci. 2004 May;66(5):529-32. doi: 10.1292/jvms.66.529. J Vet Med Sci. 2004. PMID: 15187363
-
Multiple antibody targets on herpes B glycoproteins B and D identified by screening sera of infected rhesus macaques with peptide microarrays.PLoS One. 2014 Jan 31;9(1):e86857. doi: 10.1371/journal.pone.0086857. eCollection 2014. PLoS One. 2014. PMID: 24497986 Free PMC article.
Cited by
-
Towards a comprehensive view of the herpes B virus.Front Immunol. 2023 Nov 16;14:1281384. doi: 10.3389/fimmu.2023.1281384. eCollection 2023. Front Immunol. 2023. PMID: 38035092 Free PMC article. Review.
-
Identification of unique B virus (Macacine Herpesvirus 1) epitopes of zoonotic and macaque isolates using monoclonal antibodies.PLoS One. 2017 Aug 4;12(8):e0182355. doi: 10.1371/journal.pone.0182355. eCollection 2017. PLoS One. 2017. PMID: 28783746 Free PMC article.
-
Association of the Host Immune Response with Protection Using a Live Attenuated African Swine Fever Virus Model.Viruses. 2016 Oct 22;8(10):291. doi: 10.3390/v8100291. Viruses. 2016. PMID: 27782090 Free PMC article.
-
Herpes B virus gD interaction with its human receptor--an in silico analysis approach.Theor Biol Med Model. 2014 Jun 6;11:27. doi: 10.1186/1742-4682-11-27. Theor Biol Med Model. 2014. PMID: 24902525 Free PMC article.
-
Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge.J Virol. 2016 Dec 16;91(1):e01760-16. doi: 10.1128/JVI.01760-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795430 Free PMC article.
References
-
- Agresti A, Coull B. 1998. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat 52:119–126
-
- Bernstein DI, Frenkel LM, Bryson YJ, Myers MG. 1990. Antibody response to herpes simplex virus glycoproteins gB and gD. J Med Virol 30:45–49 - PubMed
-
- Centers for Disease Control and Prevention and National Institutes of Health. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Bethesda (MD): Department of Health and Human Services.
-
- Centers for Disease Control and Prevention.[Internet] 2004. Current CLIA Regulations. [15 October 2011]. Available at: http://wwwn.cdc.gov/clia/regs/toc.aspx.
-
- Cohen JI, Davenport DS, Stewart JA, Deitchman S, Hilliard JK, Chapman LE. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine herpesvirus 1). Clin Infect Dis 35:1191–1203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
