Cartilage-specific over-expression of CCN family member 2/connective tissue growth factor (CCN2/CTGF) stimulates insulin-like growth factor expression and bone growth
- PMID: 23555635
- PMCID: PMC3610707
- DOI: 10.1371/journal.pone.0059226
Cartilage-specific over-expression of CCN family member 2/connective tissue growth factor (CCN2/CTGF) stimulates insulin-like growth factor expression and bone growth
Abstract
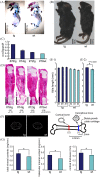
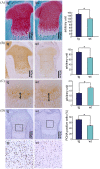
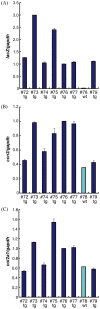
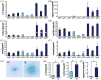
Previously we showed that CCN family member 2/connective tissue growth factor (CCN2) promotes the proliferation, differentiation, and maturation of growth cartilage cells in vitro. To elucidate the specific role and molecular mechanism of CCN2 in cartilage development in vivo, in the present study we generated transgenic mice overexpressing CCN2 and analyzed them with respect to cartilage and bone development. Transgenic mice were generated expressing a ccn2/lacZ fusion gene in cartilage under the control of the 6 kb-Col2a1-enhancer/promoter. Changes in cartilage and bone development were analyzed histologically and immunohistologically and also by micro CT. Primary chondrocytes as well as limb bud mesenchymal cells were cultured and analyzed for changes in expression of cartilage-related genes, and non-transgenic chondrocytes were treated in culture with recombinant CCN2. Newborn transgenic mice showed extended length of their long bones, increased content of proteoglycans and collagen II accumulation. Micro-CT analysis of transgenic bones indicated increases in bone thickness and mineral density. Chondrocyte proliferation was enhanced in the transgenic cartilage. In in vitro short-term cultures of transgenic chondrocytes, the expression of col2a1, aggrecan and ccn2 genes was substantially enhanced; and in long-term cultures the expression levels of these genes were further enhanced. Also, in vitro chondrogenesis was strongly enhanced. IGF-I and IGF-II mRNA levels were elevated in transgenic chondrocytes, and treatment of non-transgenic chondrocytes with recombinant CCN2 stimulated the expression of these mRNA. The addition of CCN2 to non-transgenic chondrocytes induced the phosphorylation of IGFR, and ccn2-overexpressing chondrocytes showed enhanced phosphorylation of IGFR. Our data indicates that the observed effects of CCN2 may be mediated in part by CCN2-induced overexpression of IGF-I and IGF-II. These findings indicate that CCN2-overexpression in transgenic mice accelerated the endochondral ossification processes, resulting in increased length of their long bones. Our results also indicate the possible involvement of locally enhanced IGF-I or IGF-II in this extended bone growth.
Conflict of interest statement
Figures

Similar articles
-
CCN family member 2/connective tissue growth factor (CCN2/CTGF) has anti-aging effects that protect articular cartilage from age-related degenerative changes.PLoS One. 2013 Aug 12;8(8):e71156. doi: 10.1371/journal.pone.0071156. eCollection 2013. PLoS One. 2013. PMID: 23951098 Free PMC article.
-
Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage.J Bone Miner Res. 2008 Nov;23(11):1751-64. doi: 10.1359/jbmr.080615. J Bone Miner Res. 2008. PMID: 18597638 Free PMC article.
-
CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes.Osteoarthritis Cartilage. 2008 Jul;16(7):787-95. doi: 10.1016/j.joca.2007.11.001. Epub 2008 Mar 4. Osteoarthritis Cartilage. 2008. PMID: 18289887
-
[CCN family genes in the development and differentiation of cartilage tissues].Clin Calcium. 2006 Mar;16(3):486-92. Clin Calcium. 2006. PMID: 16508133 Review. Japanese.
-
Role of CCN2/CTGF/Hcs24 in bone growth.Int Rev Cytol. 2007;257:1-41. doi: 10.1016/S0074-7696(07)57001-4. Int Rev Cytol. 2007. PMID: 17280894 Review.
Cited by
-
CTGF as a multifunctional molecule for cartilage and a potential drug for osteoarthritis.Front Endocrinol (Lausanne). 2022 Oct 17;13:1040526. doi: 10.3389/fendo.2022.1040526. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36325449 Free PMC article. Review.
-
Regulation of Long Bone Growth in Vertebrates; It Is Time to Catch Up.Endocr Rev. 2015 Dec;36(6):646-80. doi: 10.1210/er.2015-1048. Epub 2015 Oct 20. Endocr Rev. 2015. PMID: 26485225 Free PMC article. Review.
-
Multiple enhancer regions govern the transcription of CCN2 during embryonic development.J Cell Commun Signal. 2018 Mar;12(1):231-243. doi: 10.1007/s12079-017-0440-4. Epub 2017 Dec 18. J Cell Commun Signal. 2018. PMID: 29256171 Free PMC article.
-
Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery.Front Cell Dev Biol. 2020 Oct 29;8:593269. doi: 10.3389/fcell.2020.593269. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195264 Free PMC article. Review.
-
Identification of novel SHOX target genes in the developing limb using a transgenic mouse model.PLoS One. 2014 Jun 2;9(6):e98543. doi: 10.1371/journal.pone.0098543. eCollection 2014. PLoS One. 2014. PMID: 24887312 Free PMC article.
References
-
- Brigstock DR (2003) The CCN family: a new stimulus package. J Endocrinol 178: 169–175. - PubMed
-
- Takigawa M, Nishida T, Kubota S (2005) Roles of CCN2/CTGF in the cntrol of growth and regeneration. In: Perbal B, Takigawa M, editors. CCN Proteins: A new family of cell growth and differentiation regulators. London: Imperial College Press. 19–59.
-
- Kubota S, Takigawa M (2007) Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol 257: 1–41. - PubMed
-
- Takigawa M, Nakanishi T, Kubota S, Nishida T (2003) Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol 194: 256–266. - PubMed
-
- Shimo T, Nakanishi T, Kimura Y, Nishida T, Ishizeki K, et al. (1998) Inhibition of endogenous expression of connective tissue growth factor by its antisense oligonucleotide and antisense RNA suppresses proliferation and migration of vascular endothelial cells. J Biochem 124: 130–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

