Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier
- PMID: 23554500
- PMCID: PMC3643000
- DOI: 10.1523/JNEUROSCI.4051-12.2013
Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier
Abstract
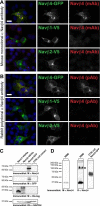
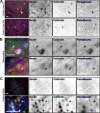
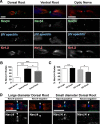
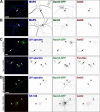
The axon initial segment (AIS) and nodes of Ranvier are the sites of action potential initiation and regeneration in axons. Although the basic molecular architectures of AIS and nodes, characterized by dense clusters of Na(+) and K(+) channels, are similar, firing patterns vary among cell types. Neuronal firing patterns are established by the collective activity of voltage-gated ion channels and can be modulated through interaction with auxiliary subunits. Here, we report the neuronal expression pattern and subcellular localization of Navβ4, the modulatory Na(+) channel subunit thought to underlie resurgent Na(+) current. Immunostaining of rat tissues revealed that Navβ4 is strongly enriched at the AIS of a select set of neuron types, including many characterized by high-frequency firing, and at nodes of Ranvier in the PNS and some nodes in the CNS. By introducing full-length and mutant GFP-tagged Navβ4 into cultured neurons, we determined that the AIS and nodal localization of Navβ4 depends on its direct interaction with Na(+) channel α subunits through an extracellular disulfide bond. Based on these results, we propose that differences in the specific composition of the Na(+) channel complexes enriched at the AIS and nodes contribute to the diverse physiologies observed among cell types.
Figures


Similar articles
-
An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier.J Neurosci. 2012 May 23;32(21):7232-43. doi: 10.1523/JNEUROSCI.5434-11.2012. J Neurosci. 2012. PMID: 22623668 Free PMC article.
-
Human Nav1.6 Channels Generate Larger Resurgent Currents than Human Nav1.1 Channels, but the Navβ4 Peptide Does Not Protect Either Isoform from Use-Dependent Reduction.PLoS One. 2015 Jul 16;10(7):e0133485. doi: 10.1371/journal.pone.0133485. eCollection 2015. PLoS One. 2015. PMID: 26182346 Free PMC article.
-
Reassembly of Excitable Domains after CNS Axon Regeneration.J Neurosci. 2016 Aug 31;36(35):9148-60. doi: 10.1523/JNEUROSCI.1747-16.2016. J Neurosci. 2016. PMID: 27581456 Free PMC article.
-
Cytoskeletal control of axon domain assembly and function.Curr Opin Neurobiol. 2016 Aug;39:116-21. doi: 10.1016/j.conb.2016.05.001. Epub 2016 May 18. Curr Opin Neurobiol. 2016. PMID: 27203619 Free PMC article. Review.
-
Neuron-glia interactions at the node of Ranvier.Results Probl Cell Differ. 2006;43:129-49. doi: 10.1007/400_014. Results Probl Cell Differ. 2006. PMID: 17068970 Review.
Cited by
-
Chemometric Models of Differential Amino Acids at the Navα and Navβ Interface of Mammalian Sodium Channel Isoforms.Molecules. 2020 Aug 3;25(15):3551. doi: 10.3390/molecules25153551. Molecules. 2020. PMID: 32756517 Free PMC article.
-
A new look at sodium channel β subunits.Open Biol. 2015 Jan;5(1):140192. doi: 10.1098/rsob.140192. Open Biol. 2015. PMID: 25567098 Free PMC article. Review.
-
More than a pore: ion channel signaling complexes.J Neurosci. 2014 Nov 12;34(46):15159-69. doi: 10.1523/JNEUROSCI.3275-14.2014. J Neurosci. 2014. PMID: 25392484 Free PMC article. Review.
-
Identification of Persistent and Resurgent Sodium Currents in Spiral Ganglion Neurons Cultured from the Mouse Cochlea.eNeuro. 2017 Nov 14;4(6):ENEURO.0303-17.2017. doi: 10.1523/ENEURO.0303-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29138759 Free PMC article.
-
Nodes of Ranvier during development and repair in the CNS.Nat Rev Neurol. 2020 Aug;16(8):426-439. doi: 10.1038/s41582-020-0375-x. Epub 2020 Jul 10. Nat Rev Neurol. 2020. PMID: 32651566 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
