A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes
- PMID: 23554169
- PMCID: PMC3628109
- DOI: 10.1002/emmm.201202023
A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes
Abstract
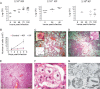
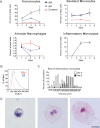
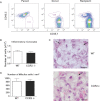
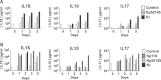
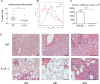
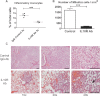
Rhinoscleroma is a human specific chronic disease characterized by the formation of granuloma in the airways, caused by the bacterium Klebsiella pneumoniae subspecies rhinoscleromatis, a species very closely related to K. pneumoniae subspecies pneumoniae. It is characterized by the appearance of specific foamy macrophages called Mikulicz cells. However, very little is known about the pathophysiological processes underlying rhinoscleroma. Herein, we characterized a murine model recapitulating the formation of Mikulicz cells in lungs and identified them as atypical inflammatory monocytes specifically recruited from the bone marrow upon K. rhinoscleromatis infection in a CCR2-independent manner. While K. pneumoniae and K. rhinoscleromatis infections induced a classical inflammatory reaction, K. rhinoscleromatis infection was characterized by a strong production of IL-10 concomitant to the appearance of Mikulicz cells. Strikingly, in the absence of IL-10, very few Mikulicz cells were observed, confirming a crucial role of IL-10 in the establishment of a proper environment leading to the maturation of these atypical monocytes. This is the first characterization of the environment leading to Mikulicz cells maturation and their identification as inflammatory monocytes.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
Similar articles
-
Rhinoscleroma pathogenesis: The type K3 capsule of Klebsiella rhinoscleromatis is a virulence factor not involved in Mikulicz cells formation.PLoS Negl Trop Dis. 2018 Jan 30;12(1):e0006201. doi: 10.1371/journal.pntd.0006201. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29381692 Free PMC article.
-
[Clinicopathologic analysis of rhinoscleroma].Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001 Feb;36(1):42-3. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2001. PMID: 12761907 Chinese.
-
Cytologic diagnosis of rhinoscleroma.Acta Cytol. 1984 Mar-Apr;28(2):139-42. Acta Cytol. 1984. PMID: 6367327
-
Social geography of Rhinoscleroma and qualitatively and quantitatively abnormal cell-mediated immunity.Infect Genet Evol. 2018 Aug;62:17-19. doi: 10.1016/j.meegid.2018.03.018. Epub 2018 Mar 22. Infect Genet Evol. 2018. PMID: 29578083 Review.
-
[Spheno-ethmoidal rhinoscleroma. Report of a case and review of the literature].Ann Otolaryngol Chir Cervicofac. 1998 May;115(2):85-8. Ann Otolaryngol Chir Cervicofac. 1998. PMID: 9765702 Review. French.
Cited by
-
Rhinoscleroma pathogenesis: The type K3 capsule of Klebsiella rhinoscleromatis is a virulence factor not involved in Mikulicz cells formation.PLoS Negl Trop Dis. 2018 Jan 30;12(1):e0006201. doi: 10.1371/journal.pntd.0006201. eCollection 2018 Jan. PLoS Negl Trop Dis. 2018. PMID: 29381692 Free PMC article.
-
Autophagy, cell death, and cytokines in K. pneumoniae infection: therapeutic perspectives.Emerg Microbes Infect. 2023 Dec;12(1):2140607. doi: 10.1080/22221751.2022.2140607. Emerg Microbes Infect. 2023. PMID: 36287114 Free PMC article. Review.
-
Rhinoscleroma with Pharyngolaryngeal Involvement Caused by Klebsiella ozaenae.Case Rep Infect Dis. 2016;2016:6536275. doi: 10.1155/2016/6536275. Epub 2016 May 12. Case Rep Infect Dis. 2016. PMID: 27293924 Free PMC article.
-
Klebsiella pneumoniae infection biology: living to counteract host defences.FEMS Microbiol Rev. 2019 Mar 1;43(2):123-144. doi: 10.1093/femsre/fuy043. FEMS Microbiol Rev. 2019. PMID: 30452654 Free PMC article. Review.
-
Comparative analysis of Klebsiella pneumoniae genomes identifies a phospholipase D family protein as a novel virulence factor.BMC Biol. 2014 May 29;12:41. doi: 10.1186/1741-7007-12-41. BMC Biol. 2014. PMID: 24885329 Free PMC article.
References
-
- Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, Boring L, Charo IF, Kunkel SL, Hogaboam CM. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J Immunol. 2000;165:2603–2611. - PubMed
-
- Blease K, Mehrad B, Lukacs NW, Kunkel SL, Standiford TJ, Hogaboam CM. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia. J Immunol. 2001;166:1832–1842. - PubMed
-
- Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. - PubMed
-
- Botelho-Nevers E, Gouriet F, Lepidi H, Couvret A, Amphoux B, Dessi P, Raoult D. Chronic nasal infection caused by Klebsiella rhinoscleromatis or Klebsiella ozaenae: two forgotten infectious diseases. Int J Infect Dis. 2007;11:423–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

