Mechanical regulation of epigenetics in vascular biology and pathobiology
- PMID: 23551392
- PMCID: PMC3822644
- DOI: 10.1111/jcmm.12031
Mechanical regulation of epigenetics in vascular biology and pathobiology
Abstract
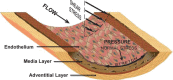
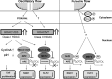
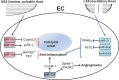
Vascular endothelial cells (ECs) and smooth muscle cells (VSMCs) are constantly exposed to haemodynamic forces, including blood flow-induced fluid shear stress and cyclic stretch from blood pressure. These forces modulate vascular cell gene expression and function and, therefore, influence vascular physiology and pathophysiology in health and disease. Epigenetics, including DNA methylation, histone modification/chromatin remodelling and RNA-based machinery, refers to the study of heritable changes in gene expression that occur without changes in the DNA sequence. The role of haemodynamic force-induced epigenetic modifications in the regulation of vascular gene expression and function has recently been elucidated. This review provides an introduction to the epigenetic concepts that relate to vascular physiology and pathophysiology. Through the studies of gene expression, cell proliferation, angiogenesis, migration and pathophysiological states, we present a conceptual framework for understanding how mechanical force-induced epigenetic modifications work to control vascular gene expression and function and, hence, the development of vascular disorders. This research contributes to our knowledge of how the mechanical environment impacts the chromatin state of ECs and VSMCs and the consequent cellular behaviours.
© 2013 The Authors. Published by Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
Similar articles
-
Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis.Curr Atheroscler Rep. 2013 May;15(5):319. Curr Atheroscler Rep. 2013. PMID: 23630979 Review.
-
Endothelial epigenetics in biomechanical stress: disturbed flow-mediated epigenomic plasticity in vivo and in vitro.Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1317-26. doi: 10.1161/ATVBAHA.115.303427. Epub 2015 Apr 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25838424 Free PMC article. Review.
-
Epigenetic drugs as pleiotropic agents in cancer treatment: biomolecular aspects and clinical applications.J Cell Physiol. 2007 Aug;212(2):330-44. doi: 10.1002/jcp.21066. J Cell Physiol. 2007. PMID: 17458893 Review.
-
Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease.Transl Res. 2019 Feb;204:19-30. doi: 10.1016/j.trsl.2018.10.002. Epub 2018 Oct 12. Transl Res. 2019. PMID: 30391475 Free PMC article. Review.
-
Epigenetics of the vascular endothelium.J Appl Physiol (1985). 2010 Sep;109(3):916-26. doi: 10.1152/japplphysiol.00131.2010. Epub 2010 Apr 22. J Appl Physiol (1985). 2010. PMID: 20413423 Review.
Cited by
-
Activated Human Adipose Tissue Transplantation Promotes Sensorimotor Recovery after Acute Spinal Cord Contusion in Rats.Cells. 2024 Jan 17;13(2):182. doi: 10.3390/cells13020182. Cells. 2024. PMID: 38247873 Free PMC article.
-
Epigenetic regulators of the revascularization response to chronic arterial occlusion.Cardiovasc Res. 2019 Mar 15;115(4):701-712. doi: 10.1093/cvr/cvz001. Cardiovasc Res. 2019. PMID: 30629133 Free PMC article. Review.
-
Sodium bicarbonate cotransporter NBCe2 gene variants increase sodium and bicarbonate transport in human renal proximal tubule cells.PLoS One. 2018 Apr 11;13(4):e0189464. doi: 10.1371/journal.pone.0189464. eCollection 2018. PLoS One. 2018. PMID: 29642240 Free PMC article.
-
Molecular and Cellular Mechanisms Involved in Aortic Wall Aneurysm Development.Diagnostics (Basel). 2023 Jan 10;13(2):253. doi: 10.3390/diagnostics13020253. Diagnostics (Basel). 2023. PMID: 36673063 Free PMC article. Review.
-
The decrease in histone methyltransferase EZH2 in response to fluid shear stress alters endothelial gene expression and promotes quiescence.Angiogenesis. 2016 Jan;19(1):9-24. doi: 10.1007/s10456-015-9485-2. Epub 2015 Sep 28. Angiogenesis. 2016. PMID: 26416763 Free PMC article.
References
-
- Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Ann NY Acad Sci. 2002;981:61–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

