Intermittent versus continuous androgen deprivation in prostate cancer
- PMID: 23550669
- PMCID: PMC3682658
- DOI: 10.1056/NEJMoa1212299
Intermittent versus continuous androgen deprivation in prostate cancer
Abstract
Background: Castration resistance occurs in most patients with metastatic hormone-sensitive prostate cancer who are receiving androgen-deprivation therapy. Replacing androgens before progression of the disease is hypothesized to prolong androgen dependence.
Methods: Men with newly diagnosed, metastatic, hormone-sensitive prostate cancer, a performance status of 0 to 2, and a prostate-specific antigen (PSA) level of 5 ng per milliliter or higher received a luteinizing hormone-releasing hormone analogue and an antiandrogen agent for 7 months. We then randomly assigned patients in whom the PSA level fell to 4 ng per milliliter or lower to continuous or intermittent androgen deprivation, with patients stratified according to prior or no prior hormonal therapy, performance status, and extent of disease (minimal or extensive). The coprimary objectives were to assess whether intermittent therapy was noninferior to continuous therapy with respect to survival, with a one-sided test with an upper boundary of the hazard ratio of 1.20, and whether quality of life differed between the groups 3 months after randomization.
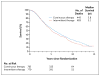
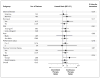
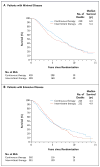
Results: A total of 3040 patients were enrolled, of whom 1535 were included in the analysis: 765 randomly assigned to continuous androgen deprivation and 770 assigned to intermittent androgen deprivation. The median follow-up period was 9.8 years. Median survival was 5.8 years in the continuous-therapy group and 5.1 years in the intermittent-therapy group (hazard ratio for death with intermittent therapy, 1.10; 90% confidence interval, 0.99 to 1.23). Intermittent therapy was associated with better erectile function and mental health (P<0.001 and P=0.003, respectively) at month 3 but not thereafter. There were no significant differences between the groups in the number of treatment-related high-grade adverse events.
Conclusions: Our findings were statistically inconclusive. In patients with metastatic hormone-sensitive prostate cancer, the confidence interval for survival exceeded the upper boundary for noninferiority, suggesting that we cannot rule out a 20% greater risk of death with intermittent therapy than with continuous therapy, but too few events occurred to rule out significant inferiority of intermittent therapy. Intermittent therapy resulted in small improvements in quality of life. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00002651.).
Figures
Comment in
-
Urological cancer: walking the tightrope of survival and quality of life with ADT.Nat Rev Clin Oncol. 2013 Jun;10(6):307-8. doi: 10.1038/nrclinonc.2013.78. Epub 2013 May 7. Nat Rev Clin Oncol. 2013. PMID: 23648827 No abstract available.
-
Androgen-deprivation therapy in men with metastatic prostate cancer: less may not necessarily be more.Asian J Androl. 2013 Jul;15(4):445-6. doi: 10.1038/aja.2013.57. Epub 2013 May 20. Asian J Androl. 2013. PMID: 23685907 Free PMC article. No abstract available.
-
Words of wisdom. Re: Intermittent versus continuous androgen deprivation in prostate cancer.Eur Urol. 2013 Dec;64(6):1014-5. doi: 10.1016/j.eururo.2013.09.031. Eur Urol. 2013. PMID: 24209449 No abstract available.
-
Re: Intermittent versus continuous androgen deprivation in prostate cancer.J Urol. 2013 Dec;190(6):2093-4. doi: 10.1016/j.juro.2013.08.102. Epub 2013 Sep 7. J Urol. 2013. PMID: 24209518 No abstract available.
-
Commentary on "Intermittent versus continuous androgen deprivation in prostate cancer." Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr., University of Michigan, Division of Hematology/Oncology, 1500 E Medical Center Dr., 7314 CC, Ann Arbor, MI. N Engl J Med 2013;368(14):1314-25.Urol Oncol. 2013 Nov;31(8):1847. doi: 10.1016/j.urolonc.2013.07.020. Urol Oncol. 2013. PMID: 24210086
-
[Prostate cancer - androgen deprivation: intermittent or continuous?].Aktuelle Urol. 2014 May;45(3):180-1. doi: 10.1055/s-0034-1383482. Epub 2014 Jun 16. Aktuelle Urol. 2014. PMID: 24932555 German. No abstract available.
-
Commentary on "Intermittent versus continuous androgen deprivation in prostate cancer." Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr, University of Michigan, Division of Hematology/Oncology, Ann Arbor, MI. N Engl J Med 2013; 368(14):1314-25. doi: 10.1056/NEJMoa1212299.Urol Oncol. 2014 Aug;32(6):936-7. doi: 10.1016/j.urolonc.2014.01.009. Urol Oncol. 2014. PMID: 25087673
Similar articles
-
Commentary on "Intermittent versus continuous androgen deprivation in prostate cancer." Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr, University of Michigan, Division of Hematology/Oncology, Ann Arbor, MI. N Engl J Med 2013; 368(14):1314-25. doi: 10.1056/NEJMoa1212299.Urol Oncol. 2014 Aug;32(6):936-7. doi: 10.1016/j.urolonc.2014.01.009. Urol Oncol. 2014. PMID: 25087673
-
Commentary on "Intermittent versus continuous androgen deprivation in prostate cancer." Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, Wilding G, Prescott S, Kanaga Sundaram S, Small EJ, Dawson NA, Donnelly BJ, Venner PM, Vaishampayan UN, Schellhammer PF, Quinn DI, Raghavan D, Ely B, Moinpour CM, Vogelzang NJ, Thompson IM Jr., University of Michigan, Division of Hematology/Oncology, 1500 E Medical Center Dr., 7314 CC, Ann Arbor, MI. N Engl J Med 2013;368(14):1314-25.Urol Oncol. 2013 Nov;31(8):1847. doi: 10.1016/j.urolonc.2013.07.020. Urol Oncol. 2013. PMID: 24210086
-
Re: Intermittent versus continuous androgen deprivation in prostate cancer.J Urol. 2013 Dec;190(6):2093-4. doi: 10.1016/j.juro.2013.08.102. Epub 2013 Sep 7. J Urol. 2013. PMID: 24209518 No abstract available.
-
Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer.Cochrane Database Syst Rev. 2014 Jun 30;2014(6):CD009266. doi: 10.1002/14651858.CD009266.pub2. Cochrane Database Syst Rev. 2014. PMID: 24979481 Free PMC article. Review.
-
Intermittent vs Continuous Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-analysis.JAMA Oncol. 2015 Dec;1(9):1261-9. doi: 10.1001/jamaoncol.2015.2895. JAMA Oncol. 2015. PMID: 26378418 Review.
Cited by
-
Integrating evolutionary dynamics into cancer therapy.Nat Rev Clin Oncol. 2020 Nov;17(11):675-686. doi: 10.1038/s41571-020-0411-1. Epub 2020 Jul 22. Nat Rev Clin Oncol. 2020. PMID: 32699310 Review.
-
Metastatic hormone-sensitive prostate cancer: How should it be treated?World J Clin Oncol. 2021 Feb 24;12(2):43-49. doi: 10.5306/wjco.v12.i2.43. World J Clin Oncol. 2021. PMID: 33680871 Free PMC article.
-
Using routinely collected data to stratify prostate cancer patients into phases of care in the United Kingdom: implications for resource allocation and the cancer survivorship programme.Br J Cancer. 2015 Apr 28;112(9):1594-602. doi: 10.1038/bjc.2014.650. Epub 2015 Mar 19. Br J Cancer. 2015. PMID: 25791873 Free PMC article.
-
Multi-institutional Analysis of the Clinical and Genomic Characteristics of Black Patients with Metastatic Hormone-Sensitive Prostate Cancer.Oncologist. 2022 Mar 11;27(3):220-227. doi: 10.1093/oncolo/oyab057. Oncologist. 2022. PMID: 35274720 Free PMC article.
-
How to Improve the Quality of Life of Patients with Prostate Cancer Treated with Hormone Therapy?Res Rep Urol. 2023 Jan 19;15:9-26. doi: 10.2147/RRU.S350793. eCollection 2023. Res Rep Urol. 2023. PMID: 36698681 Free PMC article. Review.
References
-
- Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. [Erratum, N Engl J Med 1989; 321:1420.] - PubMed
-
- Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42. - PubMed
-
- Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. - PubMed
-
- Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J Clin Oncol. 2011;29:3651–8. - PubMed
-
- Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, To M, Lawson D. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- CCSRI 015469/PHS HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA035176/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- CA63848/CA/NCI NIH HHS/United States
- CA86780/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA035176/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- CA46136/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- CA55582/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- U10 CA128567/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA180835/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- CA68183/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- N01 CA045807/CA/NCI NIH HHS/United States
- CA128567/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA063848/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- CA76132/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- CA31949/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- CA67663/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- CA77202/CA/NCI NIH HHS/United States
- U10 CA058686/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- CA46113/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- CA58686/CA/NCI NIH HHS/United States
- U10 CA086780/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA095860/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA067663/CA/NCI NIH HHS/United States
- CA95860/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- U10 CA045807/CA/NCI NIH HHS/United States
- U10 CA068183/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
