Transcriptome Profiling Following Neuronal and Glial Expression of ALS-Linked SOD1 in Drosophila
- PMID: 23550139
- PMCID: PMC3618356
- DOI: 10.1534/g3.113.005850
Transcriptome Profiling Following Neuronal and Glial Expression of ALS-Linked SOD1 in Drosophila
Abstract
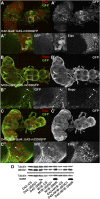
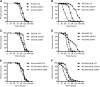
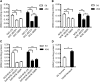
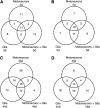
Amyotrophic lateral sclerosis (ALS) generally is a late-onset neurodegenerative disease. Mutations in the Cu/Zn superoxide dismutase 1 (SOD1) gene account for approximately 20% of familial ALS and 2% of all ALS cases. Although a number of hypotheses have been proposed to explain mutant SOD1 toxicity, the molecular mechanisms of the disease remain unclear. SOD1-linked ALS is thought to function in a non-cell-autonomous manner such that motoneurons are critical for the onset, and glia contribute to progression of the disease. Recently, it has been shown in Drosophila melanogaster that expression of human SOD1 in a subset of neuronal cells causes synaptic transmission defects, modified motor function, and altered sensitivity to compounds that induce oxidative stress. Here we used the Gal4-UAS (Upstream Activation Sequence) system to further characterize flies expressing wild-type Drosophila SOD1 (dSOD1) and the mutant human SOD1G85R (G85R) allele in motoneurons and glia. Cell-specific expression of both dSOD1 and G85R was found to influence lifespan, affect sensitivity to hydrogen peroxide, and alter lipid peroxidation levels. To better understand the genetic consequences of G85R expression in motoneurons and glia, we conducted microarray analysis of both young flies (5 days old) and old flies (45 days old) expressing G85R selectively in motoneurons or glia and concurrently in motoneurons and glia. Results from this microarray experiment identified candidate genes for further investigation and may help elucidate the individual and combined contributions of motoneurons and glia in ALS.
Keywords: ALS; Drosophila; SOD1; glia; motoneuron.
Copyright © 2013 Kumimoto et al.
Figures

Similar articles
-
ALS-linked SOD1 in glial cells enhances ß-N-Methylamino L-Alanine (BMAA)-induced toxicity in Drosophila.F1000Res. 2012 Nov 9;1:47. doi: 10.12688/f1000research.1-47.v1. eCollection 2012. F1000Res. 2012. PMID: 24627764 Free PMC article.
-
Circuit Dysfunction in SOD1-ALS Model First Detected in Sensory Feedback Prior to Motor Neuron Degeneration Is Alleviated by BMP Signaling.J Neurosci. 2019 Mar 20;39(12):2347-2364. doi: 10.1523/JNEUROSCI.1771-18.2019. Epub 2019 Jan 18. J Neurosci. 2019. PMID: 30659087 Free PMC article.
-
Up-regulation of cholesterol synthesis pathways and limited neurodegeneration in a knock-in Sod1 mutant mouse model of ALS.bioRxiv [Preprint]. 2023 May 5:2023.05.05.539444. doi: 10.1101/2023.05.05.539444. bioRxiv. 2023. PMID: 37205335 Free PMC article. Preprint.
-
Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis.J Physiol Paris. 2006 Mar-May;99(2-3):211-20. doi: 10.1016/j.jphysparis.2005.12.014. Epub 2006 Jan 30. J Physiol Paris. 2006. PMID: 16448809 Review.
-
SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS.Adv Biol Regul. 2016 Jan;60:95-104. doi: 10.1016/j.jbior.2015.10.006. Epub 2015 Oct 31. Adv Biol Regul. 2016. PMID: 26563614 Review.
Cited by
-
Dysregulation of antimicrobial peptide expression distinguishes Alzheimer's disease from normal aging.Aging (Albany NY). 2020 Jan 6;12(1):690-706. doi: 10.18632/aging.102650. Epub 2020 Jan 6. Aging (Albany NY). 2020. PMID: 31907335 Free PMC article.
-
Investigating cell death mechanisms in amyotrophic lateral sclerosis using transcriptomics.Front Cell Neurosci. 2013 Dec 17;7:259. doi: 10.3389/fncel.2013.00259. Front Cell Neurosci. 2013. PMID: 24381542 Free PMC article. Review.
-
Meta-analysis of Genetic Modifiers Reveals Candidate Dysregulated Pathways in Amyotrophic Lateral Sclerosis.Neuroscience. 2019 Jan 1;396:A3-A20. doi: 10.1016/j.neuroscience.2018.10.033. Neuroscience. 2019. PMID: 30594291 Free PMC article.
-
A fruitful endeavor: modeling ALS in the fruit fly.Brain Res. 2015 May 14;1607:47-74. doi: 10.1016/j.brainres.2014.09.064. Epub 2014 Oct 5. Brain Res. 2015. PMID: 25289585 Free PMC article. Review.
-
RNA Dysregulation in Amyotrophic Lateral Sclerosis.Front Genet. 2019 Jan 22;9:712. doi: 10.3389/fgene.2018.00712. eCollection 2018. Front Genet. 2019. PMID: 30723494 Free PMC article. Review.
References
-
- Afifi A. K., Aleu F. P., Goodgold J., Mackay B., 1966. Ultrastructure of atrophic muscle in amyotrophic lateral sclerosis. Neurology 16: 475–481 - PubMed
-
- Alexianu M. E., Kozovska M., Appel S. H., 2001. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 57: 1282–1289 - PubMed
-
- Bier E., Jan L. Y., Jan Y. N., 1990. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev. 4: 190–203 - PubMed
-
- Boillee S., Vande Velde C., Cleveland D. W., 2006a ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52: 39–59 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
