Analysis of the oligomeric state and transactivation potential of TAp73α
- PMID: 23538419
- PMCID: PMC3705593
- DOI: 10.1038/cdd.2013.23
Analysis of the oligomeric state and transactivation potential of TAp73α
Abstract
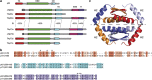
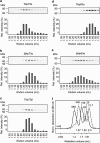
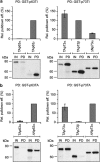
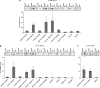
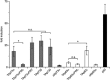
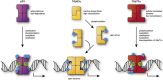
The proteins p73 and p63 are members of the p53 protein family and are involved in important developmental processes. Their high sequence identity with the tumor suppressor p53 has suggested that they act as tumor suppressors as well. While p63 has a crucial role in the maintenance of epithelial stem cells and in the quality control of oocytes without a clear role as a tumor suppressor, p73's tumor suppressor activity is well documented. In a recent study we have shown that the transcriptional activity of TAp63α, the isoform responsible for the quality control in oocytes, is regulated by its oligomeric state. The protein forms an inactive, dimeric and compact conformation in resting oocytes, while the detection of DNA damage leads to the formation of an active, tetrameric and open conformation. p73 shows a high sequence identity to p63, including those domains that are crucial in stabilizing its inactive state, thus suggesting that p73's activity might be regulated by its oligomeric state as well. Here, we have investigated the oligomeric state of TAp73α by size exclusion chromatography and detailed domain interaction mapping, and show that in contrast to p63, TAp73α is a constitutive open tetramer. However, its transactivation potential depends on the cellular background and the promoter context. These results imply that the regulation of p73's transcriptional activity might be more closely related to p53 than to p63.
Figures
Comment in
-
p73 - constitutively open for business.Cell Death Differ. 2013 Aug;20(8):972-3. doi: 10.1038/cdd.2013.56. Cell Death Differ. 2013. PMID: 23832148 Free PMC article. No abstract available.
Similar articles
-
p73 - constitutively open for business.Cell Death Differ. 2013 Aug;20(8):972-3. doi: 10.1038/cdd.2013.56. Cell Death Differ. 2013. PMID: 23832148 Free PMC article. No abstract available.
-
Regulation of the Activity in the p53 Family Depends on the Organization of the Transactivation Domain.Structure. 2018 Aug 7;26(8):1091-1100.e4. doi: 10.1016/j.str.2018.05.013. Epub 2018 Jun 28. Structure. 2018. PMID: 30099987
-
Impact of cadmium, cobalt and nickel on sequence-specific DNA binding of p63 and p73 in vitro and in cells.Biochem Biophys Res Commun. 2015 Jan 2;456(1):29-34. doi: 10.1016/j.bbrc.2014.11.027. Epub 2014 Nov 18. Biochem Biophys Res Commun. 2015. PMID: 25446071
-
Structural diversity of p63 and p73 isoforms.Cell Death Differ. 2022 May;29(5):921-937. doi: 10.1038/s41418-022-00975-4. Epub 2022 Mar 21. Cell Death Differ. 2022. PMID: 35314772 Free PMC article. Review.
-
p63/p73 in the control of cell cycle and cell death.Exp Cell Res. 2012 Jul 1;318(11):1285-90. doi: 10.1016/j.yexcr.2012.01.023. Epub 2012 Feb 3. Exp Cell Res. 2012. PMID: 22326462 Review.
Cited by
-
Whole-genome cartography of p53 response elements ranked on transactivation potential.BMC Genomics. 2015 Jun 17;16(1):464. doi: 10.1186/s12864-015-1643-9. BMC Genomics. 2015. PMID: 26081755 Free PMC article.
-
p63 threonine phosphorylation signals the interaction with the WW domain of the E3 ligase Itch.Cell Cycle. 2014;13(20):3207-17. doi: 10.4161/15384101.2014.951285. Cell Cycle. 2014. PMID: 25485500 Free PMC article.
-
p73 regulates basal and starvation-induced liver metabolism in vivo.Oncotarget. 2015 Oct 20;6(32):33178-90. doi: 10.18632/oncotarget.5090. Oncotarget. 2015. PMID: 26375672 Free PMC article.
-
p73 - constitutively open for business.Cell Death Differ. 2013 Aug;20(8):972-3. doi: 10.1038/cdd.2013.56. Cell Death Differ. 2013. PMID: 23832148 Free PMC article. No abstract available.
-
Unlocking the Gateway: The Spatio-Temporal Dynamics of the p53 Family Driven by the Nuclear Pores and Its Implication for the Therapeutic Approach in Cancer.Int J Mol Sci. 2024 Jul 7;25(13):7465. doi: 10.3390/ijms25137465. Int J Mol Sci. 2024. PMID: 39000572 Free PMC article. Review.
References
-
- Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. - PubMed
-
- Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nat Rev Mol Cell Biol. 2011;12:259–265. - PubMed
-
- Ollmann M, Young LM, Di Como CJ, Karim F, Belvin M, Robertson S, et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell. 2000;101:91–101. - PubMed
-
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

