Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice
- PMID: 23536373
- PMCID: PMC4013901
- DOI: 10.1095/biolreprod.112.106104
Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice
Abstract
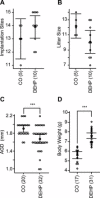
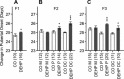
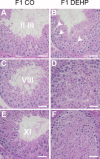
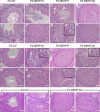
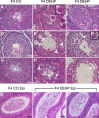
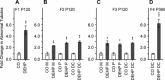
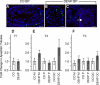
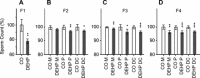
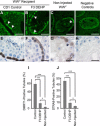
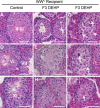
Recent evidence has linked human phthalate exposure to abnormal reproductive and hormonal effects. Phthalates are plasticizers that confer flexibility and transparency to plastics, but they readily contaminate the body and the environment. In this study, timed pregnant CD1 outbred mice were treated with di-(2-ethylhexyl) phthalate (DEHP) from Embryonic Day 7 (E7) to E14. The subsequent generation (F1) offspring were then bred to produce the F2, F3, and F4 offspring, without any further DEHP treatment. This exposure scheme disrupted testicular germ cell association and decreased sperm count and motility in F1 to F4 offspring. By spermatogonial transplantation techniques, the exposure scheme also disrupted spermatogonial stem cell (SSC) function of F3 offspring. The W/W(V) recipient testes transplanted with F3 offspring germ cells from the DEHP-treated group had a dramatically lower percentage of donor germ cell-derived spermatogenic recovery in seminiferous tubules when compared to the recipient testes transplanted with CD1 control germ cells. Further characterization showed that the major block of donor germ cell-derived spermatogenesis was before the appearance of undifferentiated spermatogonia. Interestingly, the testes transplanted with the F3 offspring germ cells from the DEHP-treated group, when regenerated, replicated testis morphology similar to that observed in the testes from the F1 to F3 offspring of the DEHP-treated group, suggesting that the germ cell disorganization phenotype originates from the stem cells of F3 offspring. In conclusion, embryonic exposure to DEHP was found to disrupt testicular germ cell organization and SSC function in a transgenerational manner.
Keywords: DEHP; environmental contaminants and toxicants; male germ cells; spermatogenesis; spermatogonial stem cells; testis; transgenerational.
Figures

Similar articles
-
Influence of di-(2-ethylhexyl)phthalate on fetal testicular development by oral administration to pregnant rats.J Toxicol Sci. 2005 Aug;30(3):175-94. doi: 10.2131/jts.30.175. J Toxicol Sci. 2005. PMID: 16141652
-
Transgenerational Effects of Di(2-Ethylhexyl) Phthalate on Anogenital Distance, Sperm Functions and DNA Methylation in Rat Offspring.Int J Mol Sci. 2021 Apr 16;22(8):4131. doi: 10.3390/ijms22084131. Int J Mol Sci. 2021. PMID: 33923623 Free PMC article.
-
Maternal exposure to Di-(2-ethylhexyl) phthalate (DEHP) activates the PI3K/Akt/mTOR signaling pathway in F1 and F2 generation adult mouse testis.Exp Cell Res. 2020 Sep 15;394(2):112151. doi: 10.1016/j.yexcr.2020.112151. Epub 2020 Jun 23. Exp Cell Res. 2020. PMID: 32589889
-
Spermatogenesis by Sisyphus: proliferating stem germ cells fail to repopulate the testis after 'irreversible' injury.Adv Exp Med Biol. 2001;500:421-8. doi: 10.1007/978-1-4615-0667-6_64. Adv Exp Med Biol. 2001. PMID: 11764975 Review.
-
Seminiferous cord formation and germ-cell programming: epigenetic transgenerational actions of endocrine disruptors.Ann N Y Acad Sci. 2005 Dec;1061:18-32. doi: 10.1196/annals.1336.004. Ann N Y Acad Sci. 2005. PMID: 16467254 Free PMC article. Review.
Cited by
-
The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes.Nutrients. 2023 Nov 3;15(21):4657. doi: 10.3390/nu15214657. Nutrients. 2023. PMID: 37960310 Free PMC article. Review.
-
Single-cell transcriptome dissection of the toxic impact of Di (2-ethylhexyl) phthalate on primordial follicle assembly.Theranostics. 2021 Mar 5;11(10):4992-5009. doi: 10.7150/thno.55006. eCollection 2021. Theranostics. 2021. PMID: 33754040 Free PMC article.
-
A systematic review on the adverse health effects of di-2-ethylhexyl phthalate.Environ Sci Pollut Res Int. 2016 Dec;23(24):24642-24693. doi: 10.1007/s11356-016-7648-3. Epub 2016 Oct 6. Environ Sci Pollut Res Int. 2016. PMID: 27714658 Review.
-
Phthalate esters affect maturation and function of primate testis tissue ectopically grafted in mice.Mol Cell Endocrinol. 2014 Dec;398(1-2):89-100. doi: 10.1016/j.mce.2014.10.004. Epub 2014 Oct 27. Mol Cell Endocrinol. 2014. PMID: 25450860 Free PMC article.
-
Phthalate pollution in an Amazonian rainforest.Environ Sci Pollut Res Int. 2016 Aug;23(16):16865-72. doi: 10.1007/s11356-016-7141-z. Epub 2016 Jul 2. Environ Sci Pollut Res Int. 2016. PMID: 27372101
References
-
- Halden RU. Plastics and health risks. Annu Rev Public Health 2010; 31: 179 194. - PubMed
-
- CERHR. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di (Ethylhexyl) Phthalate (DEHP). Research Triangle Park, North Carolina: National Institutes of Health; 2006: i III76. - PubMed
-
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 2004; 112: 331 338. - PMC - PubMed
-
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod 2007; 22: 688 695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

