Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy
- PMID: 23536077
- PMCID: PMC6705058
- DOI: 10.1523/JNEUROSCI.3659-12.2013
Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy
Abstract
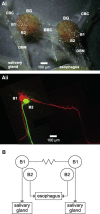
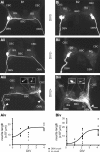
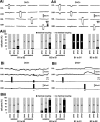
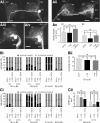
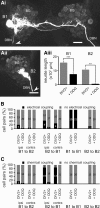
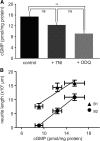
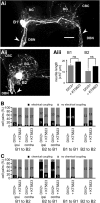
Nitric oxide (NO) is an important signaling molecule with a variety of functions in the CNS, including a potential role in modulating neuronal growth and synapse formation. In the present study, we used tractable, identified neurons in the CNS of the pond snail Lymnaea stagnalis to study the role of endogenous NO signaling in neuronal growth and synaptic remodeling after nerve injury. Axonal damage of L. stagnalis neurons B1 and B2 induces extensive central growth of neurites that is accompanied by changes in existing electrical connections, the transient formation of novel electrical connections, and the formation of a novel excitatory chemical synapse from B2 to B1 neurons. Partial chronic inhibition of endogenous NO synthesis reduces neurite growth in NO-synthase-expressing B2, but has only minor effects on NOS-negative B1 neurons. Chronic application of an NO donor while inhibiting endogenous NO synthesis rescues neurite extension in B2 neurons and boosts growth of B1 neurons. Blocking soluble guanylate cyclase activity completely suppresses neurite extension and synaptic remodeling after nerve crush, demonstrating the importance of cGMP in these processes. Interestingly, inhibition of cGMP-dependent protein kinase only suppresses chemical synapse formation without effects on neuronal growth and electrical synapse remodeling. We conclude that NO signaling via cGMP is an important modulator of both neurite growth and synaptic remodeling after nerve crush. However, differential effects of cGMP-dependent protein kinase inhibition on neurite growth and synaptic remodeling suggest that these effects are mediated by separate signaling pathways.
Figures

Similar articles
-
Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism.J Neurosci Res. 1997 Feb 15;47(4):427-39. J Neurosci Res. 1997. PMID: 9057136
-
Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108-15 cells.Neuroendocrinology. 2002 Jan;75(1):70-81. doi: 10.1159/000048222. Neuroendocrinology. 2002. PMID: 11810036
-
Nitric oxide as intracellular modulator: internal production of NO increases neuronal excitability via modulation of several ionic conductances.Eur J Neurosci. 2012 Nov;36(10):3333-43. doi: 10.1111/j.1460-9568.2012.08260.x. Epub 2012 Aug 23. Eur J Neurosci. 2012. PMID: 22913584
-
Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects.Rev Neurosci. 2006;17(3):309-57. doi: 10.1515/revneuro.2006.17.3.309. Rev Neurosci. 2006. PMID: 16878402 Review.
-
Traumatic injury of the spinal cord and nitric oxide.Prog Brain Res. 2007;161:171-83. doi: 10.1016/S0079-6123(06)61011-X. Prog Brain Res. 2007. PMID: 17618976 Review.
Cited by
-
Prohibitin S-Nitrosylation Is Required for the Neuroprotective Effect of Nitric Oxide in Neuronal Cultures.J Neurosci. 2020 Apr 15;40(16):3142-3151. doi: 10.1523/JNEUROSCI.1804-19.2020. Epub 2020 Mar 9. J Neurosci. 2020. PMID: 32152200 Free PMC article.
-
Near-infrared light-triggered NO release for spinal cord injury repair.Sci Adv. 2020 Sep 25;6(39):eabc3513. doi: 10.1126/sciadv.abc3513. Print 2020 Sep. Sci Adv. 2020. PMID: 32978153 Free PMC article.
-
Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects.Front Physiol. 2015 May 11;6:139. doi: 10.3389/fphys.2015.00139. eCollection 2015. Front Physiol. 2015. PMID: 26029110 Free PMC article. Review.
-
In Situ Forming of Nitric Oxide and Electric Stimulus for Nerve Therapy by Wireless Chargeable Gold Yarn-Dynamos.Adv Sci (Weinh). 2023 Nov;10(33):e2303566. doi: 10.1002/advs.202303566. Epub 2023 Oct 22. Adv Sci (Weinh). 2023. PMID: 37867218 Free PMC article.
-
Endogenous Conjugation of Biomimetic Dinitrosyl Iron Complex with Protein Vehicles for Oral Delivery of Nitric Oxide to Brain and Activation of Hippocampal Neurogenesis.JACS Au. 2021 Jun 7;1(7):998-1013. doi: 10.1021/jacsau.1c00160. eCollection 2021 Jul 26. JACS Au. 2021. PMID: 34467346 Free PMC article.
References
-
- Benjamin PR, Rose RM. Central generation of bursting in the feeding system of the snail, Lymnaea stagnalis. J Exp Biol. 1979;80:93–118. - PubMed
-
- Benjamin PR, Winlow W. The distribution of 3 wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea stagnalis (L) Comp Biochem Phys A. 1981;70:293–307.
-
- Benjamin PR, Rose RM, Slade CT, Lacy MG. Morphology of identified neurones in the buccal ganglia of Lymnaea stagnalis. J Exp Biol. 1979;80:119–135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
