Cellular microbiology and molecular ecology of Legionella-amoeba interaction
- PMID: 23535283
- PMCID: PMC3710333
- DOI: 10.4161/viru.24290
Cellular microbiology and molecular ecology of Legionella-amoeba interaction
Abstract
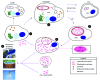
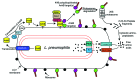
Legionella pneumophila is an aquatic organism that interacts with amoebae and ciliated protozoa as the natural hosts, and this interaction plays a central role in bacterial ecology and infectivity. Upon transmission to humans, L. pneumophila infect and replicate within alveolar macrophages causing pneumonia. Intracellular proliferation of L. pneumophila within the two evolutionarily distant hosts is facilitated by bacterial exploitation of evolutionarily conserved host processes that are targeted by bacterial protein effectors injected into the host cell by the Dot/Icm type VIB translocation system. Although cysteine is semi-essential for humans and essential for amoeba, it is a metabolically favorable source of carbon and energy generation by L. pneumophila. To counteract host limitation of cysteine, L. pneumophila utilizes the AnkB Dot/Icm-translocated F-box effector to promote host proteasomal degradation of polyubiquitinated proteins within amoebae and human cells. Evidence indicates ankB and other Dot/Icm-translocated effector genes have been acquired through inter-kingdom horizontal gene transfer.
Keywords: Ankyrin; Ankyrin B; Dot/Icm; Legionnaire; RelA; SpoT; cysteine; effectors AnkB; farnesylation; pneumophila; polyubiquitin; ppGpp; prenylation; proteasomes.
Figures
Similar articles
-
Exploitation of evolutionarily conserved amoeba and mammalian processes by Legionella.Trends Microbiol. 2012 Jun;20(6):299-306. doi: 10.1016/j.tim.2012.03.005. Epub 2012 Apr 9. Trends Microbiol. 2012. PMID: 22494803 Free PMC article. Review.
-
Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae.Infect Immun. 2010 May;78(5):2079-88. doi: 10.1128/IAI.01450-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194593 Free PMC article.
-
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa.PLoS Pathog. 2009 Dec;5(12):e1000704. doi: 10.1371/journal.ppat.1000704. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041211 Free PMC article.
-
Amoeba host-Legionella synchronization of amino acid auxotrophy and its role in bacterial adaptation and pathogenic evolution.Environ Microbiol. 2014 Feb;16(2):350-8. doi: 10.1111/1462-2920.12290. Epub 2013 Oct 21. Environ Microbiol. 2014. PMID: 24112119 Free PMC article. Review.
-
A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa.Mol Microbiol. 2008 Nov;70(4):908-23. doi: 10.1111/j.1365-2958.2008.06453.x. Epub 2008 Sep 22. Mol Microbiol. 2008. PMID: 18811729 Free PMC article.
Cited by
-
A Legionella pneumophila amylase is essential for intracellular replication in human macrophages and amoebae.Sci Rep. 2018 Apr 20;8(1):6340. doi: 10.1038/s41598-018-24724-1. Sci Rep. 2018. PMID: 29679057 Free PMC article.
-
Investigating the Molecular Genetic Basis of Cytoplasmic Sex Determination Caused by Wolbachia Endosymbionts in Terrestrial Isopods.Genes (Basel). 2018 Jun 8;9(6):290. doi: 10.3390/genes9060290. Genes (Basel). 2018. PMID: 29890648 Free PMC article.
-
Paradoxical Pro-inflammatory Responses by Human Macrophages to an Amoebae Host-Adapted Legionella Effector.Cell Host Microbe. 2020 Apr 8;27(4):571-584.e7. doi: 10.1016/j.chom.2020.03.003. Epub 2020 Mar 27. Cell Host Microbe. 2020. PMID: 32220647 Free PMC article.
-
Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila.Front Cell Infect Microbiol. 2014 Aug 26;4:111. doi: 10.3389/fcimb.2014.00111. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25207263 Free PMC article. Review. No abstract available.
-
Ecology of Legionella pneumophila biofilms: The link between transcriptional activity and the biphasic cycle.Biofilm. 2024 Mar 30;7:100196. doi: 10.1016/j.bioflm.2024.100196. eCollection 2024 Jun. Biofilm. 2024. PMID: 38601816 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
