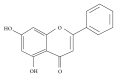
5,7-Dihydroxyflavone Enhances the Apoptosis-Inducing Potential of TRAIL in Human Tumor Cells via Regulation of Apoptosis-Related Proteins
- PMID: 23533482
- PMCID: PMC3600283
- DOI: 10.1155/2013/434709
5,7-Dihydroxyflavone Enhances the Apoptosis-Inducing Potential of TRAIL in Human Tumor Cells via Regulation of Apoptosis-Related Proteins
Abstract
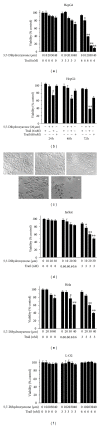
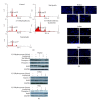
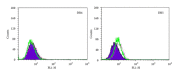
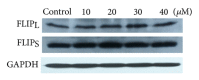
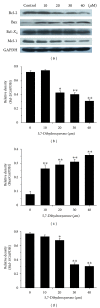
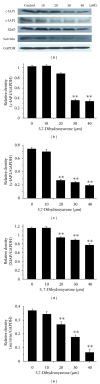
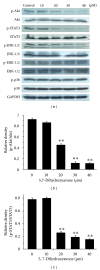
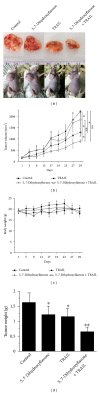
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising candidate for the treatment of cancer, because it preferentially induces apoptosis in numerous cancer cells with little or no effect on normal cells. 5,7-Dihydroxyflavone is a dietary flavonoid commonly found in many plants. Here we show that the combined treatment with 5,7-dihydroxyflavone and TRAIL at subtoxic concentrations induced strong apoptotic response in human hepatocarcinoma HepG2 cells, acute leukemia Jurkat T cells, and cervical carcinoma HeLa cells. We further investigated the mechanisms by which 5,7-dihydroxyflavone augments TRAIL-induced apoptosis in HepG2 cells. 5,7-Dihydroxyflavone up-regulated the expression of pro-apoptotic protein Bax, attenuated the expression of anti-apoptotic proteins Bcl-2, Mcl-1, and IAPs, and reduced the phosphorylation levels of Akt and STAT3, weakening the anti-apoptotic signals thus facilitating the process of apoptosis. Moreover, 5,7-dihydroxyflavone and TRAIL were well tolerated in mice, and the combination of 5,7-dihydroxyflavone and TRAIL reduced tumor burden in vivo in a HepG2 tumor xenograft model. Interestingly, 5,7-dihydroxyflavone-mediated sensitization to TRAIL-induced cell death was not observed in normal human hepatocytes L-O2. These results suggest that the 5,7-dihydroxyflavone in combination with TRAIL might be used for cancer prevention and/or therapy.
Figures
Similar articles
-
The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation.Cancer Res. 2007 Oct 1;67(19):9490-500. doi: 10.1158/0008-5472.CAN-07-0598. Cancer Res. 2007. PMID: 17909059
-
α-Hispanolol sensitizes hepatocellular carcinoma cells to TRAIL-induced apoptosis via death receptor up-regulation.Toxicol Appl Pharmacol. 2015 Aug 1;286(3):168-77. doi: 10.1016/j.taap.2015.04.012. Epub 2015 Apr 28. Toxicol Appl Pharmacol. 2015. PMID: 25930665
-
Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation.Int J Oncol. 2013 Jul;43(1):329-37. doi: 10.3892/ijo.2013.1926. Epub 2013 May 1. Int J Oncol. 2013. PMID: 23636231
-
Caffeic acid phenethyl ester enhances TRAIL-mediated apoptosis via CHOP-induced death receptor 5 upregulation in hepatocarcinoma Hep3B cells.Mol Cell Biochem. 2016 Jul;418(1-2):13-20. doi: 10.1007/s11010-016-2726-x. Epub 2016 Jun 3. Mol Cell Biochem. 2016. PMID: 27260301
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Apoptotic Pathway as the Therapeutic Target for Anticancer Traditional Chinese Medicines.Front Pharmacol. 2019 Jul 12;10:758. doi: 10.3389/fphar.2019.00758. eCollection 2019. Front Pharmacol. 2019. PMID: 31354479 Free PMC article. Review.
-
Pharmacological Effects of Polyphenol Phytochemicals on the JAK-STAT Signaling Pathway.Front Pharmacol. 2021 Sep 3;12:716672. doi: 10.3389/fphar.2021.716672. eCollection 2021. Front Pharmacol. 2021. PMID: 34539403 Free PMC article. Review.
-
Established Human Cell Lines as Models to Study Anti-leukemic Effects of Flavonoids.Curr Genomics. 2017 Feb;18(1):3-26. doi: 10.2174/1389202917666160803165447. Curr Genomics. 2017. PMID: 28503087 Free PMC article.
-
TRAIL-Sensitizing Effects of Flavonoids in Cancer.Int J Mol Sci. 2023 Nov 22;24(23):16596. doi: 10.3390/ijms242316596. Int J Mol Sci. 2023. PMID: 38068921 Free PMC article. Review.
-
Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy.Cells. 2020 Jun 11;9(6):1451. doi: 10.3390/cells9061451. Cells. 2020. PMID: 32545187 Free PMC article. Review.
References
-
- Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. - PubMed
-
- Koschny R, Walczak H, Ganten TM. The promise of TRAIL—potential and risks of a novel anticancer therapy. Journal of Molecular Medicine. 2007;85(9):923–935. - PubMed
-
- Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22(53):8628–8633. - PubMed
-
- Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nature Reviews Cancer. 2008;8(10):782–798. - PubMed
-
- Rieger J, Frank B, Weller M, Wick W. Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. Cellular Physiology and Biochemistry. 2007;20(1-4):23–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

