Kaposi sarcoma-associated herpesvirus g protein-coupled receptor enhances endothelial cell survival in part by upregulation of bcl-2
- PMID: 23532945
- PMCID: PMC3603191
Kaposi sarcoma-associated herpesvirus g protein-coupled receptor enhances endothelial cell survival in part by upregulation of bcl-2
Abstract
Background: Kaposi sarcoma-associated herpesvirus (KSHV) encoded G protein-coupled receptor (vGPCR) is a constitutively active lytic phase protein with significant homology to the human interleukin-8 receptor. vGPCR is necessary and sufficient to induce angiogenesis as well as the spindle cell proliferation characteristic of Kaposi sarcoma (KS) lesions. We previously demonstrated that Bcl-2, an antiapoptotic protein, is upregulated in KS lesions. The aim of this study was to determine if vGPCR enhances endothelial cell survival through upregulation of Bcl-2 expression and to elucidate the signaling pathways involved.
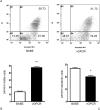
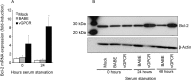
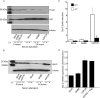
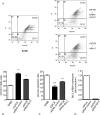
Methods: Primary human umbilical vein endothelial cells were transduced with a recombinant retrovirus expressing vGPCR and then subjected to serum starvation. Cell viability and apoptosis were analyzed by fluorescence-activated cell sorting. Bcl-2 expression was determined by real-time quantitative reverse transcription polymerase chain reaction and immunoblotting. Specific pharmacological inhibitors of phosphatidylinositol 3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) were employed to elucidate the signaling pathways involved. Bcl-2 expression was knocked down using small interfering RNA (siRNA).
Results: Endothelial cells expressing vGPCR showed increased survival after serum starvation and upregulation of Bcl-2 messenger RNA (mRNA) and protein. The vGPCR-induced increases in both Bcl-2 mRNA and protein levels were dependent on PI3K signaling but not on mTOR. Moreover, siRNA inhibition of Bcl-2 resulted in significant abrogation of the observed vGPCR-mediated cell survival advantage.
Conclusions: Taken together, the results demonstrate that Bcl-2 is a mediator of vGPCR-induced endothelial cell survival and is a downstream effector of Akt in this process.
Keywords: Bcl-2 protein; G protein–coupled receptor; Kaposi sarcoma; human herpesvirus 8; phosphatidylinositol 3-kinase.
Conflict of interest statement
Figures
Similar articles
-
Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor.J Biol Chem. 2006 Apr 21;281(16):11332-46. doi: 10.1074/jbc.M512199200. Epub 2006 Feb 13. J Biol Chem. 2006. PMID: 16476737
-
The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia.Blood. 2004 Nov 1;104(9):2903-11. doi: 10.1182/blood-2003-12-4436. Epub 2004 Jul 1. Blood. 2004. PMID: 15231571
-
Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi's sarcoma.PLoS One. 2011 Apr 29;6(4):e19103. doi: 10.1371/journal.pone.0019103. PLoS One. 2011. PMID: 21559457 Free PMC article.
-
Cell cycle arrest and apoptosis induced by 1α,25(OH)2D3 and TX 527 in Kaposi sarcoma is VDR dependent.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:197-200. doi: 10.1016/j.jsbmb.2013.11.014. Epub 2013 Dec 5. J Steroid Biochem Mol Biol. 2014. PMID: 24316429 Review.
-
Akt/TSC/mTOR activation by the KSHV G protein-coupled receptor: emerging insights into the molecular oncogenesis and treatment of Kaposi's sarcoma.Cell Cycle. 2007 Feb 15;6(4):438-43. doi: 10.4161/cc.6.4.3843. Epub 2007 Feb 12. Cell Cycle. 2007. PMID: 17329974 Review.
Cited by
-
Mesenchymal-to-endothelial transition in Kaposi sarcoma: a histogenetic hypothesis based on a case series and literature review.PLoS One. 2013 Aug 6;8(8):e71530. doi: 10.1371/journal.pone.0071530. Print 2013. PLoS One. 2013. PMID: 23936513 Free PMC article.
-
Signaling Molecules in Posttransplantation Cancer.Clin Lab Med. 2019 Mar;39(1):171-183. doi: 10.1016/j.cll.2018.10.006. Epub 2018 Dec 18. Clin Lab Med. 2019. PMID: 30709505 Free PMC article. Review.
-
The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside.Virology. 2015 May;479-480:568-77. doi: 10.1016/j.virol.2015.02.040. Epub 2015 Mar 20. Virology. 2015. PMID: 25798530 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994 Dec 16;266(5192):1865–1869. - PubMed
-
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000 Apr 6;342(14):1027–1038. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995 May 4;332(18):1186–1191. - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995 Aug 15;86(4):1276–1280. - PubMed
-
- Boshoff C, Schulz TF, Kennedy MM, et al. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995 Dec;1(12):1274–1278. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
