Microtubule dynamic instability: a new model with coupled GTP hydrolysis and multistep catastrophe
- PMID: 23532586
- PMCID: PMC3677417
- DOI: 10.1002/bies.201200131
Microtubule dynamic instability: a new model with coupled GTP hydrolysis and multistep catastrophe
Erratum in
- Bioessays. 2013 Jun;35(6):579
Abstract
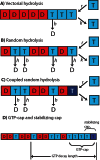
A key question in understanding microtubule dynamics is how GTP hydrolysis leads to catastrophe, the switch from slow growth to rapid shrinkage. We first provide a review of the experimental and modeling literature, and then present a new model of microtubule dynamics. We demonstrate that vectorial, random, and coupled hydrolysis mechanisms are not consistent with the dependence of catastrophe on tubulin concentration and show that, although single-protofilament models can explain many features of dynamics, they do not describe catastrophe as a multistep process. Finally, we present a new combined (coupled plus random hydrolysis) multiple-protofilament model that is a simple, analytically solvable generalization of a single-protofilament model. This model accounts for the observed lifetimes of growing microtubules, the delay to catastrophe following dilution and describes catastrophe as a multistep process.
Copyright © 2013 WILEY Periodicals, Inc.
Figures


Similar articles
-
Dynamic instability of microtubules: Monte Carlo simulation and application to different types of microtubule lattice.Biophys J. 1993 Aug;65(2):578-96. doi: 10.1016/S0006-3495(93)81091-9. Biophys J. 1993. PMID: 8218889 Free PMC article.
-
Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues.Science. 2008 Nov 28;322(5906):1353-6. doi: 10.1126/science.1165401. Epub 2008 Oct 16. Science. 2008. PMID: 18927356
-
Microtubule catastrophe from protofilament dynamics.Phys Rev E Stat Nonlin Soft Matter Phys. 2013 Sep;88(3):032717. doi: 10.1103/PhysRevE.88.032717. Epub 2013 Sep 30. Phys Rev E Stat Nonlin Soft Matter Phys. 2013. PMID: 24125304
-
Microtubule catastrophe and rescue.Curr Opin Cell Biol. 2013 Feb;25(1):14-22. doi: 10.1016/j.ceb.2012.09.006. Epub 2012 Oct 22. Curr Opin Cell Biol. 2013. PMID: 23092753 Free PMC article. Review.
-
Dynamic instability 30 years later: complexities in microtubule growth and catastrophe.Mol Biol Cell. 2015 Apr 1;26(7):1207-10. doi: 10.1091/mbc.E13-10-0594. Mol Biol Cell. 2015. PMID: 25823928 Free PMC article. Review.
Cited by
-
AMXI-5001, a novel dual parp1/2 and microtubule polymerization inhibitor for the treatment of human cancers.Am J Cancer Res. 2020 Aug 1;10(8):2649-2676. eCollection 2020. Am J Cancer Res. 2020. PMID: 32905466 Free PMC article.
-
The size of the EB cap determines instantaneous microtubule stability.Elife. 2016 Apr 6;5:e13470. doi: 10.7554/eLife.13470. Elife. 2016. PMID: 27050486 Free PMC article.
-
Interface-acting nucleotide controls polymerization dynamics at microtubule plus- and minus-ends.Elife. 2024 Jan 5;12:RP89231. doi: 10.7554/eLife.89231. Elife. 2024. PMID: 38180336 Free PMC article.
-
Fine structure and dynamics of EB3 binding zones on microtubules in fibroblast cells.Mol Biol Cell. 2019 Aug 1;30(17):2105-2114. doi: 10.1091/mbc.E18-11-0723. Epub 2019 May 29. Mol Biol Cell. 2019. PMID: 31141458 Free PMC article.
-
Lattice defects induced by microtubule-stabilizing agents exert a long-range effect on microtubule growth by promoting catastrophes.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2112261118. doi: 10.1073/pnas.2112261118. Proc Natl Acad Sci U S A. 2021. PMID: 34916292 Free PMC article.
References
-
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42. - PubMed
-
- Gardner MK, Zanic M, Gell C, Bormuth V, et al. Depolymerizing kinesins Kip3 and MCAK shape cellular microtubule architecture by differential control of catastrophe. Cell. 2011;147:1092–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

