Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte-collagen interactions
- PMID: 23530036
- PMCID: PMC3675607
- DOI: 10.1074/jbc.M112.431528
Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte-collagen interactions
Abstract
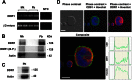
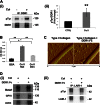
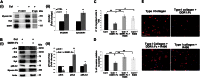
Growing evidence demonstrates that extracellular matrices regulate many aspects of megakaryocyte (MK) development; however, among the different extracellular matrix receptors, integrin α2β1 and glycoprotein VI are the only collagen receptors studied in platelets and MKs. In this study, we demonstrate the expression of the novel collagen receptor discoidin domain receptor 1 (DDR1) by human MKs at both mRNA and protein levels and provide evidence of DDR1 involvement in the regulation of MK motility on type I collagen through a mechanism based on the activity of SHP1 phosphatase and spleen tyrosine kinase (Syk). Specifically, we demonstrated that inhibition of DDR1 binding to type I collagen, preserving the engagement of the other collagen receptors, glycoprotein VI, α2β1, and LAIR-1, determines a decrease in MK migration due to the reduction in SHP1 phosphatase activity and consequent increase in the phosphorylation level of its main substrate Syk. Consistently, inhibition of Syk activity restored MK migration on type I collagen. In conclusion, we report the expression and function of a novel collagen receptor on human MKs, and we point out that an increasing level of complexity is necessary to better understand MK-collagen interactions in the bone marrow environment.
Keywords: Bone Marrow; Discoidin Domain Receptor 1; Extracellular Matrix; Hematopoiesis; Megakaryocytes; Phosphatase; Receptors.
Figures


Similar articles
-
Collagen I regulates the self-renewal of mouse embryonic stem cells through α2β1 integrin- and DDR1-dependent Bmi-1.J Cell Physiol. 2011 Dec;226(12):3422-32. doi: 10.1002/jcp.22697. J Cell Physiol. 2011. PMID: 21344393
-
A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration.Mol Biol Cell. 2006 Jun;17(6):2839-52. doi: 10.1091/mbc.e05-11-1068. Epub 2006 Apr 12. Mol Biol Cell. 2006. PMID: 16611743 Free PMC article.
-
Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors.Cell Mol Life Sci. 2011 Nov;68(22):3757-70. doi: 10.1007/s00018-011-0676-8. Epub 2011 Apr 17. Cell Mol Life Sci. 2011. PMID: 21499918 Free PMC article.
-
Discoidin domain receptor 1: a new class of receptor regulating leukocyte-collagen interaction.Immunol Res. 2005;31(3):219-30. doi: 10.1385/IR:31:3:219. Immunol Res. 2005. PMID: 15888913 Review.
-
Collagen recognition and transmembrane signalling by discoidin domain receptors.Biochim Biophys Acta. 2013 Oct;1834(10):2187-94. doi: 10.1016/j.bbapap.2012.10.014. Epub 2012 Nov 2. Biochim Biophys Acta. 2013. PMID: 23128141 Free PMC article. Review.
Cited by
-
Dynamins 2 and 3 control the migration of human megakaryocytes by regulating CXCR4 surface expression and ITGB1 activity.Blood Adv. 2018 Dec 11;2(23):3540-3552. doi: 10.1182/bloodadvances.2018021923. Blood Adv. 2018. PMID: 30538113 Free PMC article.
-
Imaging platelet biogenesis in vivo.Res Pract Thromb Haemost. 2018 Jun 10;2(3):461-468. doi: 10.1002/rth2.12112. eCollection 2018 Jul. Res Pract Thromb Haemost. 2018. PMID: 30046750 Free PMC article. Review.
-
The BRAF-inhibitor PLX4720 inhibits CXCL8 secretion in BRAFV600E mutated and normal thyroid cells: a further anti-cancer effect of BRAF-inhibitors.Sci Rep. 2019 Mar 13;9(1):4390. doi: 10.1038/s41598-019-40818-w. Sci Rep. 2019. PMID: 30867499 Free PMC article.
-
The Molecular Interaction of Collagen with Cell Receptors for Biological Function.Polymers (Basel). 2022 Feb 23;14(5):876. doi: 10.3390/polym14050876. Polymers (Basel). 2022. PMID: 35267698 Free PMC article. Review.
-
A new path to platelet production through matrix sensing.Haematologica. 2017 Jul;102(7):1150-1160. doi: 10.3324/haematol.2016.161562. Epub 2017 Apr 14. Haematologica. 2017. PMID: 28411253 Free PMC article.
References
-
- Avecilla S. T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., Jin D. K., Dias S., Zhang F., Hartman T. E., Hackett N. R., Crystal R. G., Witte L., Hicklin D. J., Bohlen P., Eaton D., Lyden D., de Sauvage F., Rafii S. (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10, 64–71 - PubMed
-
- Junt T., Schulze H., Chen Z., Massberg S., Goerge T., Krueger A., Wagner D. D., Graf T., Italiano J. E., Jr., Shivdasani R. A., von Andrian U. H. (2007) Dynamic visualization of thrombopoiesis within bone marrow. Science 317, 1767–1770 - PubMed
-
- Nilsson S. K., Debatis M. E., Dooner M. S., Madri J. A., Quesenberry P. J., Becker P. S. (1998) Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 46, 371–377 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

