Agonistic TAM-163 antibody targeting tyrosine kinase receptor-B: applying mechanistic modeling to enable preclinical to clinical translation and guide clinical trial design
- PMID: 23529133
- PMCID: PMC4169031
- DOI: 10.4161/mabs.23826
Agonistic TAM-163 antibody targeting tyrosine kinase receptor-B: applying mechanistic modeling to enable preclinical to clinical translation and guide clinical trial design
Abstract
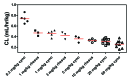
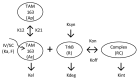
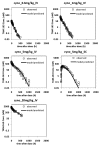
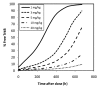
TAM-163, an agonist monoclonal antibody targeting tyrosine receptor kinase-B (TrkB), is currently being investigated as a potential body weight modulatory agent in humans. To support the selection of the dose range for the first-in-human (FIH) trial of TAM-163, we conducted a mechanistic analysis of the pharmacokinetic (PK) and pharmacodynamic (PD) data (e.g., body weight gain) obtained in lean cynomolgus and obese rhesus monkeys following single doses ranging from 0.3 to 60 mg/kg. A target-mediated drug disposition (TMDD) model was used to describe the observed nonlinear PK and Emax approach was used to describe the observed dose-dependent PD effect. The TMDD model development was supported by the experimental determination of the binding affinity constant (9.4 nM) and internalization rate of the drug-target complex (2.08 h(-1)). These mechanistic analyses enabled linking of exposure, target (TrkB) coverage, and pharmacological activity (e.g., PD) in monkeys, and indicated that ≥ 38% target coverage (time-average) was required to achieve significant body weight gain in monkeys. Based on the scaling of the TMDD model from monkeys to humans and assuming similar relationship between the target coverage and pharmacological activity between monkey and humans, subcutaneous (SC) doses of 1 and 15 mg/kg in humans were projected to be the minimally and the fully pharmacologically active doses, respectively. Based on the minimal anticipated biological effect level (MABEL) approach for starting dose selection, the dose of 0.05 mg/kg (3 mg for a 60 kg human) SC was recommended as the starting dose for FIH trials, because at this dose level<10% target coverage was projected at Cmax (and all other time points). This study illustrates a rational mechanistic approach for the selection of FIH dose range for a therapeutic protein with a complex model of action.
Keywords: Cachexia; PK/PD modeling; TAM-163; TMDD; TrkB; antibody; body weight; modeling; pharmacodynamics; pharmacokinetics.
Figures


Similar articles
-
Target-Mediated Drug Disposition Pharmacokinetic/Pharmacodynamic Model-Informed Dose Selection for the First-in-Human Study of AVB-S6-500.Clin Transl Sci. 2020 Jan;13(1):204-211. doi: 10.1111/cts.12706. Epub 2019 Oct 25. Clin Transl Sci. 2020. PMID: 31599479 Free PMC article. Clinical Trial.
-
Target-mediated drug disposition and prolonged liver accumulation of a novel humanized anti-CD81 monoclonal antibody in cynomolgus monkeys.MAbs. 2013 Sep-Oct;5(5):776-86. doi: 10.4161/mabs.25642. Epub 2013 Jul 8. MAbs. 2013. PMID: 23924796 Free PMC article.
-
Activation of TrkB with TAM-163 results in opposite effects on body weight in rodents and non-human primates.PLoS One. 2013 May 20;8(5):e62616. doi: 10.1371/journal.pone.0062616. Print 2013. PLoS One. 2013. PMID: 23700410 Free PMC article.
-
Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method.Eur J Pharm Sci. 2021 Mar 1;158:105584. doi: 10.1016/j.ejps.2020.105584. Epub 2020 Oct 9. Eur J Pharm Sci. 2021. PMID: 33039565 Review.
-
The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies.Curr Opin Biotechnol. 2009 Dec;20(6):722-9. doi: 10.1016/j.copbio.2009.10.013. Epub 2009 Nov 5. Curr Opin Biotechnol. 2009. PMID: 19896825 Review.
Cited by
-
Recent Advances in Translational Pharmacokinetics and Pharmacodynamics Prediction of Therapeutic Antibodies Using Modeling and Simulation.Pharmaceuticals (Basel). 2022 Apr 22;15(5):508. doi: 10.3390/ph15050508. Pharmaceuticals (Basel). 2022. PMID: 35631335 Free PMC article. Review.
-
Allometric scaling of therapeutic monoclonal antibodies in preclinical and clinical settings.MAbs. 2021 Jan-Dec;13(1):1964935. doi: 10.1080/19420862.2021.1964935. MAbs. 2021. PMID: 34530672 Free PMC article. Review.
-
Quantitative analysis of target coverage and germinal center response by a CXCL13 neutralizing antibody in a T-dependent mouse immunization model.Pharm Res. 2014 Mar;31(3):635-48. doi: 10.1007/s11095-013-1185-2. Epub 2013 Nov 5. Pharm Res. 2014. PMID: 24190631
-
Estimation of Clearance and Bioavailability of Therapeutic Monoclonal Antibodies from Only Subcutaneous Injection Data in Humans Based on Comprehensive Analysis of Clinical Data.Clin Pharmacokinet. 2021 Oct;60(10):1325-1334. doi: 10.1007/s40262-021-01023-z. Epub 2021 May 6. Clin Pharmacokinet. 2021. PMID: 33954956 Free PMC article.
-
Determination of the starting dose in the first-in-human clinical trials with monoclonal antibodies: a systematic review of papers published between 1990 and 2013.Drug Des Devel Ther. 2016 Dec 8;10:4005-4016. doi: 10.2147/DDDT.S121520. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27994442 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
