M2e-displaying virus-like particles with associated RNA promote T helper 1 type adaptive immunity against influenza A
- PMID: 23527091
- PMCID: PMC3601086
- DOI: 10.1371/journal.pone.0059081
M2e-displaying virus-like particles with associated RNA promote T helper 1 type adaptive immunity against influenza A
Abstract
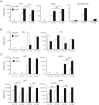
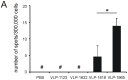
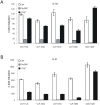
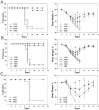
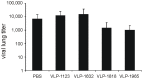
The ectodomain of influenza A matrix protein 2 (M2e) is a candidate for a universal influenza A vaccine. We used recombinant Hepatitis B core antigen to produce virus-like particles presenting M2e (M2e-VLPs). We produced the VLPs with and without entrapped nucleic acids and compared their immunogenicity and protective efficacy. Immunization of BALB/c mice with M2e-VLPs containing nucleic acids induced a stronger, Th1-biased antibody response compared to particles lacking nucleic acids. The former also induced a stronger M2e-specific CD4(+) T cell response, as determined by ELISPOT. Mice vaccinated with alum-adjuvanted M2e-VLPs containing the nucleic acid-binding domain were better protected against influenza A virus challenge than mice vaccinated with similar particles lacking this domain, as deduced from the loss in body weight following challenge with X47 (H3N2) or PR/8 virus. Challenge of mice that had been immunized with M2e-VLPs with or without nucleic acids displayed significantly lower mortality, morbidity and lung virus titers than control-immunized groups. We conclude that nucleic acids present in M2e-VLPs correlate with improved immune protection.
Trial registration: ClinicalTrials.gov NCT00819013.
Conflict of interest statement
Figures

Similar articles
-
Development of a candidate influenza vaccine based on virus-like particles displaying influenza M2e peptide into the immunodominant region of hepatitis B core antigen: Broad protective efficacy of particles carrying four copies of M2e.Vaccine. 2015 Jun 26;33(29):3398-406. doi: 10.1016/j.vaccine.2015.04.073. Epub 2015 May 11. Vaccine. 2015. PMID: 25976545
-
Vaccine Efficacy Induced by 2009 Pandemic H1N1 Virus-Like Particles Differs from that Induced by Split Influenza Virus.Immunol Invest. 2020 Oct;49(7):781-793. doi: 10.1080/08820139.2019.1694539. Epub 2019 Nov 27. Immunol Invest. 2020. PMID: 31774021
-
Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse.Virol J. 2007 Oct 31;4:118. doi: 10.1186/1743-422X-4-118. Virol J. 2007. PMID: 17974006 Free PMC article.
-
Towards a universal influenza vaccine: volunteer virus challenge studies in quarantine to speed the development and subsequent licensing.Br J Clin Pharmacol. 2013 Aug;76(2):210-6. doi: 10.1111/bcp.12146. Br J Clin Pharmacol. 2013. PMID: 23617282 Free PMC article. Review.
-
Virus-like particles as antigenic nanomaterials for inducing protective immune responses in the lung.Nanomedicine (Lond). 2014;9(12):1857-68. doi: 10.2217/nnm.14.107. Nanomedicine (Lond). 2014. PMID: 25325241 Free PMC article. Review.
Cited by
-
Non-carrier nanoparticles adjuvant modular protein vaccine in a particle-dependent manner.PLoS One. 2015 Mar 10;10(3):e0117203. doi: 10.1371/journal.pone.0117203. eCollection 2015. PLoS One. 2015. PMID: 25756283 Free PMC article.
-
Influenza-Host Interplay and Strategies for Universal Vaccine Development.Vaccines (Basel). 2020 Sep 20;8(3):548. doi: 10.3390/vaccines8030548. Vaccines (Basel). 2020. PMID: 32962304 Free PMC article. Review.
-
Bacteriophage T4 Vaccine Platform for Next-Generation Influenza Vaccine Development.Front Immunol. 2021 Oct 12;12:745625. doi: 10.3389/fimmu.2021.745625. eCollection 2021. Front Immunol. 2021. PMID: 34712234 Free PMC article.
-
Whole-Chain Tick Saliva Proteins Presented on Hepatitis B Virus Capsid-Like Particles Induce High-Titered Antibodies with Neutralizing Potential.PLoS One. 2015 Sep 9;10(9):e0136180. doi: 10.1371/journal.pone.0136180. eCollection 2015. PLoS One. 2015. PMID: 26352137 Free PMC article.
-
Potential recombinant vaccine against influenza A virus based on M2e displayed on nodaviral capsid nanoparticles.Int J Nanomedicine. 2015 Apr 2;10:2751-63. doi: 10.2147/IJN.S77405. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25897220 Free PMC article.
References
-
- Thompson WW, Comanor L, Shay DK (2006) Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. The Journal of infectious diseases 194 Suppl 2: S82–91. - PubMed
-
- Nichol KL, Treanor JJ (2006) Vaccines for seasonal and pandemic influenza. J Infect Dis 194 Suppl 2: S111–118. - PubMed
-
- Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, et al.. (2012) Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet infectious diseases. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

