Telomerase and telomere length in pulmonary fibrosis
- PMID: 23526226
- PMCID: PMC3824037
- DOI: 10.1165/rcmb.2012-0514OC
Telomerase and telomere length in pulmonary fibrosis
Abstract
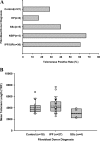
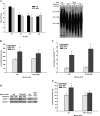
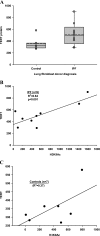
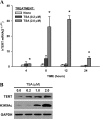
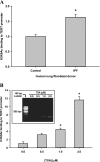
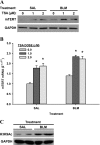
In addition to its expression in stem cells and many cancers, telomerase activity is transiently induced in murine bleomycin (BLM)-induced pulmonary fibrosis with increased levels of telomerase transcriptase (TERT) expression, which is essential for fibrosis. To extend these observations to human chronic fibrotic lung disease, we investigated the expression of telomerase activity in lung fibroblasts from patients with interstitial lung diseases (ILDs), including idiopathic pulmonary fibrosis (IPF). The results showed that telomerase activity was induced in more than 66% of IPF lung fibroblast samples, in comparison with less than 29% from control samples, some of which were obtained from lung cancer resections. Less than 4% of the human IPF lung fibroblast samples exhibited shortened telomeres, whereas less than 6% of peripheral blood leukocyte samples from patients with IPF or hypersensitivity pneumonitis demonstrated shortened telomeres. Moreover, shortened telomeres in late-generation telomerase RNA component knockout mice did not exert a significant effect on BLM-induced pulmonary fibrosis. In contrast, TERT knockout mice exhibited deficient fibrosis that was independent of telomere length. Finally, TERT expression was up-regulated by a histone deacetylase inhibitor, while the induction of TERT in lung fibroblasts was associated with the binding of acetylated histone H3K9 to the TERT promoter region. These findings indicate that significant telomerase induction was evident in fibroblasts from fibrotic murine lungs and a majority of IPF lung samples, whereas telomere shortening was not a common finding in the human blood and lung fibroblast samples. Notably, the animal studies indicated that the pathogenesis of pulmonary fibrosis was independent of telomere length.
Figures

Similar articles
-
Telomerase deficiency does not alter bleomycin-induced fibrosis in mice.Exp Lung Res. 2012 Apr;38(3):124-34. doi: 10.3109/01902148.2012.658148. Exp Lung Res. 2012. PMID: 22394286 Free PMC article.
-
Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence.J Biol Chem. 2019 May 31;294(22):8861-8871. doi: 10.1074/jbc.RA118.006615. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000627 Free PMC article.
-
Telomerase treatment prevents lung profibrotic pathologies associated with physiological aging.J Cell Biol. 2020 Oct 5;219(10):e202002120. doi: 10.1083/jcb.202002120. J Cell Biol. 2020. PMID: 32777016 Free PMC article.
-
[A concise review of telomere and telomerase-related genetic markers in fibrotic lung diseases].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020 Dec 20;38(12):952-956. doi: 10.3760/cma.j.cn121094-20200305-00104. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020. PMID: 33406566 Review. Chinese.
-
Telomeres in Interstitial Lung Disease.J Clin Med. 2021 Mar 30;10(7):1384. doi: 10.3390/jcm10071384. J Clin Med. 2021. PMID: 33808277 Free PMC article. Review.
Cited by
-
Epigenetics in immune-mediated pulmonary diseases.Clin Rev Allergy Immunol. 2013 Dec;45(3):314-30. doi: 10.1007/s12016-013-8398-3. Clin Rev Allergy Immunol. 2013. PMID: 24242359 Review.
-
Compromised DNA repair is responsible for diabetes-associated fibrosis.EMBO J. 2020 Jun 2;39(11):e103477. doi: 10.15252/embj.2019103477. Epub 2020 Apr 27. EMBO J. 2020. PMID: 32338774 Free PMC article.
-
Potent telomerase activators from a novel sapogenin via biotransformation utilizing Camarosporium laburnicola, an endophytic fungus.Microb Cell Fact. 2023 Apr 6;22(1):66. doi: 10.1186/s12934-023-02069-3. Microb Cell Fact. 2023. PMID: 37024895 Free PMC article.
-
Optimization of biotransformation processes of Camarosporium laburnicola to improve production yields of potent telomerase activators.Microb Cell Fact. 2024 Jul 10;23(1):196. doi: 10.1186/s12934-024-02468-0. Microb Cell Fact. 2024. PMID: 38987741 Free PMC article.
-
Lung epithelial stem cells and their niches: Fgf10 takes center stage.Fibrogenesis Tissue Repair. 2014 May 8;7:8. doi: 10.1186/1755-1536-7-8. eCollection 2014. Fibrogenesis Tissue Repair. 2014. PMID: 24891877 Free PMC article. Review.
References
-
- Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. - PubMed
-
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. - PubMed
-
- Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. - PubMed
-
- Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, et al. Reconstitution of human telomerase with the template RNA component HTR and the catalytic protein subunit HTRT. Nat Genet. 1997;17:498–502. - PubMed
-
- Nozaki Y, Liu T, Hatano K, Gharaee-Kermani M, Phan SH. Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am J Respir Cell Mol Biol. 2000;23:460–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL028737/HL/NHLBI NIH HHS/United States
- HL077297/HL/NHLBI NIH HHS/United States
- R01 HL074882/HL/NHLBI NIH HHS/United States
- AR05084/AR/NIAMS NIH HHS/United States
- HL052285/HL/NHLBI NIH HHS/United States
- R01 HL052285/HL/NHLBI NIH HHS/United States
- R01 HL077297/HL/NHLBI NIH HHS/United States
- P01 HL091775/HL/NHLBI NIH HHS/United States
- R01 HL028737/HL/NHLBI NIH HHS/United States
- HL028737/HL/NHLBI NIH HHS/United States
- HL091775/HL/NHLBI NIH HHS/United States
- HL074882/HL/NHLBI NIH HHS/United States
- R01 HL089249/HL/NHLBI NIH HHS/United States
- R01 HL112880/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

