Graft-versus-host disease is enhanced by selective CD73 blockade in mice
- PMID: 23520507
- PMCID: PMC3592842
- DOI: 10.1371/journal.pone.0058397
Graft-versus-host disease is enhanced by selective CD73 blockade in mice
Abstract
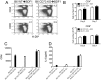
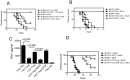
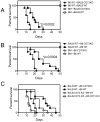
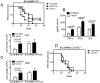
CD73 functions as an ecto-5'-nucleotidase to produce extracellular adenosine that has anti-inflammatory and immunosuppressive activity. We here demonstrate that CD73 helps control graft-versus-host disease (GVHD) in mouse models. Survival of wild-type (WT) recipients of either allogeneic donor naïve CD73 knock-out (KO) or WT T cells was similar suggesting that donor naïve T cell CD73 did not contribute to GVHD. By contrast, donor CD73 KO CD4(+)CD25(+) regulatory T cells (Treg) had significantly impaired ability to mitigate GVHD mortality compared to WT Treg, suggesting that CD73 on Treg is critical for GVHD protection. However, compared to donor CD73, recipient CD73 is more effective in limiting GVHD. Pharmacological blockade of A2A receptor exacerbated GVHD in WT recipients, but not in CD73 KO recipients, suggesting that A2 receptor signaling is primarily implicated in CD73-mediated GVHD protection. Moreover, pharmacological blockade of CD73 enzymatic activity induced stronger alloreactive T cell activity, worsened GVHD and enhanced the graft-versus-leukemia (GVL) effect. These findings suggest that both donor and recipient CD73 protects against GVHD but also limits GVL effects. Thus, either enhancing or blocking CD73 activity has great potential clinical application in allogeneic bone marrow transplants.
Conflict of interest statement
Figures



Similar articles
-
Deficiency of CD73/ecto-5'-nucleotidase in mice enhances acute graft-versus-host disease.Blood. 2012 May 10;119(19):4554-64. doi: 10.1182/blood-2011-09-375899. Epub 2012 Jan 18. Blood. 2012. PMID: 22262774 Free PMC article.
-
[Extracellular adenosine is a therapeutic target for limiting graft-versus-host disease and enhancing the graft-versus-tumor effect against hematopoietic malignancy].Yakugaku Zasshi. 2014;134(10):1021-7. doi: 10.1248/yakushi.14-00184. Yakugaku Zasshi. 2014. PMID: 25274211 Review. Japanese.
-
Differential Effect of MyD88 Signal in Donor T Cells on Graft-versus-Leukemia Effect and Graft-versus-Host Disease after Experimental Allogeneic Stem Cell Transplantation.Mol Cells. 2015 Nov;38(11):966-74. doi: 10.14348/molcells.2015.0158. Epub 2015 Nov 10. Mol Cells. 2015. PMID: 26552489 Free PMC article.
-
Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14711-6. doi: 10.1073/pnas.1308209110. Epub 2013 Aug 20. Proc Natl Acad Sci U S A. 2013. PMID: 23964122 Free PMC article.
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
Cited by
-
Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease?Biomedicines. 2017 Jul 1;5(3):39. doi: 10.3390/biomedicines5030039. Biomedicines. 2017. PMID: 28671556 Free PMC article. Review.
-
Purinergic Profiling of Regulatory T-cells in Patients With Episodic Migraine.Front Cell Neurosci. 2018 Sep 25;12:326. doi: 10.3389/fncel.2018.00326. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30319363 Free PMC article.
-
Novel elucidation and treatment of pancreatic chronic graft-versus-host disease in mice.R Soc Open Sci. 2018 Oct 17;5(10):181067. doi: 10.1098/rsos.181067. eCollection 2018 Oct. R Soc Open Sci. 2018. PMID: 30473850 Free PMC article.
-
Chip-Based Sensing of the Intercellular Transfer of Cell Surface Proteins: Regulation by the Metabolic State.Biomedicines. 2021 Oct 13;9(10):1452. doi: 10.3390/biomedicines9101452. Biomedicines. 2021. PMID: 34680568 Free PMC article.
-
Purinergic signalling and immune cells.Purinergic Signal. 2014 Dec;10(4):529-64. doi: 10.1007/s11302-014-9427-2. Epub 2014 Oct 29. Purinergic Signal. 2014. PMID: 25352330 Free PMC article. Review.
References
-
- Shlomchik WD (2007) Graft-versus-host disease. Nat Rev Immunol 7: 340–352. - PubMed
-
- Ferrara JL, Levy R, Chao NJ (1999) Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant 5: 347–356. - PubMed
-
- Welniak LA, Blazar BR, Murphy WJ (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 25: 139–170. - PubMed
-
- Holler E (2002) Cytokines, viruses, and graft-versus-host disease. Curr Opin Hematol 9: 479–484. - PubMed
-
- van den Brink MR, Burakoff SJ (2002) Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol 2: 273–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

