Evaluation of Head Movement Periodicity and Irregularity during Locomotion of Caenorhabditis elegans
- PMID: 23518645
- PMCID: PMC3604732
- DOI: 10.3389/fnbeh.2013.00020
Evaluation of Head Movement Periodicity and Irregularity during Locomotion of Caenorhabditis elegans
Abstract
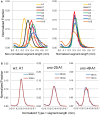
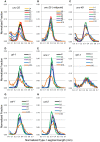
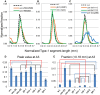
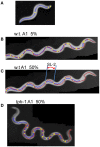
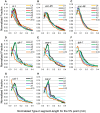
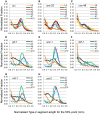
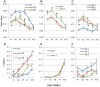
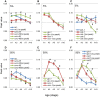
Caenorhabditis elegans is suitable for studying the nervous system, which controls behavior. C. elegans shows sinusoidal locomotion on an agar plate. The head moves not only sinusoidally but also more complexly, which reflects regulation of the head muscles by the nervous system. The head movement becomes more irregular with senescence. To date, the head movement complexity has not been quantitatively analyzed. We propose two simple methods for evaluation of the head movement regularity on an agar plate using image analysis. The methods calculate metrics that are a measure of how the head end movement is correlated with body movement. In the first method, the length along the trace of the head end on the agar plate between adjacent intersecting points of the head trace and the quasi-midline of the head trace, which was made by sliding an averaging window of 1/2 the body wavelength, was obtained. Histograms of the lengths showed periodic movement of the head and deviation from it. In the second method, the intersections between the trace of the head end and the trace of the 5 (near the pharynx) or 50% (the mid-body) point from the head end in the centerline length of the worm image were marked. The length of the head trace between adjacent intersections was measured, and a histogram of the lengths was produced. The histogram for the 5% point showed deviation of the head end movement from the movement near the pharynx. The histogram for the 50% point showed deviation of the head movement from the sinusoidal movement of the body center. Application of these methods to wild type and several mutant strains enabled evaluation of their head movement periodicity and irregularity, and revealed a difference in the age-dependence of head movement irregularity between the strains. A set of five parameters obtained from the histograms reliably identifies differences in head movement between strains.
Keywords: behavior; image analysis; mutant; nematode; senescence.
Figures

Similar articles
-
Computer-driven automatic identification of locomotion states in Caenorhabditis elegans.J Neurosci Methods. 2006 Oct 30;157(2):355-63. doi: 10.1016/j.jneumeth.2006.05.002. Epub 2006 Jun 5. J Neurosci Methods. 2006. PMID: 16750860
-
An automated system for measuring parameters of nematode sinusoidal movement.BMC Genet. 2005 Feb 7;6:5. doi: 10.1186/1471-2156-6-5. BMC Genet. 2005. PMID: 15698479 Free PMC article.
-
Locomotion of C. elegans: a piecewise-harmonic curvature representation of nematode behavior.PLoS One. 2012;7(7):e40121. doi: 10.1371/journal.pone.0040121. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792224 Free PMC article.
-
Automatic tracking, feature extraction and classification of C elegans phenotypes.IEEE Trans Biomed Eng. 2004 Oct;51(10):1811-20. doi: 10.1109/TBME.2004.831532. IEEE Trans Biomed Eng. 2004. PMID: 15490828
-
Clues to basis of exploratory behaviour of the C. elegans snout from head somatotropy.Philos Trans R Soc Lond B Biol Sci. 2018 Sep 10;373(1758):20170367. doi: 10.1098/rstb.2017.0367. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 30201833 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

