Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo
- PMID: 23516621
- PMCID: PMC3597602
- DOI: 10.1371/journal.pone.0059276
Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo
Abstract
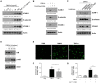
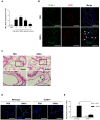
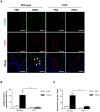
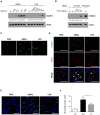
Escherichia coli, as one of the gut microbiota, can evoke severe inflammatory diseases including peritonitis and sepsis. Gram-negative bacteria including E. coli constitutively release nano-sized outer membrane vesicles (OMVs). Although E. coli OMVs can induce the inflammatory responses without live bacteria, the effect of E. coli OMVs in vivo on endothelial cell function has not been previously elucidated. In this study, we show that bacteria-free OMVs increased the expression of endothelial intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1, and enhanced the leukocyte binding on human microvascular endothelial cells in vitro. Inhibition of NF-κB and TLR4 reduced the expression of cell adhesion molecules in vitro. OMVs given intraperitoneally to the mice induced ICAM-1 expression and neutrophil sequestration in the lung endothelium, and the effects were reduced in ICAM-1(-/-) and TLR4(-/-) mice. When compared to free lipopolysaccharide, OMVs were more potent in inducing both ICAM-1 expression as well as leukocyte adhesion in vitro, and ICAM-1 expression and neutrophil sequestration in the lungs in vivo. This study shows that OMVs potently up-regulate functional cell adhesion molecules via NF-κB- and TLR4-dependent pathways, and that OMVs are more potent than free lipopolysaccharide.
Conflict of interest statement
Figures

Similar articles
-
Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells.J Surg Res. 2013 Sep;184(1):458-66. doi: 10.1016/j.jss.2013.05.035. Epub 2013 Jun 2. J Surg Res. 2013. PMID: 23800440
-
Outer Membrane Vesicles Derived From Escherichia coli Regulate Neutrophil Migration by Induction of Endothelial IL-8.Front Microbiol. 2018 Oct 11;9:2268. doi: 10.3389/fmicb.2018.02268. eCollection 2018. Front Microbiol. 2018. PMID: 30369908 Free PMC article.
-
Leukocyte adhesion molecule and chemokine production through lipoteichoic acid recognition by toll-like receptor 2 in cultured human lymphatic endothelium.Cell Tissue Res. 2008 Aug;333(2):237-52. doi: 10.1007/s00441-008-0625-5. Epub 2008 Jun 4. Cell Tissue Res. 2008. PMID: 18523807
-
Aurintricarboxylic acid inhibits the nuclear factor-κB-dependent expression of intercellular cell adhesion molecule-1 and endothelial cell selectin on activated human endothelial cells.Blood Coagul Fibrinolysis. 2011 Mar;22(2):132-9. doi: 10.1097/MBC.0b013e32834356b6. Blood Coagul Fibrinolysis. 2011. PMID: 21245742
-
Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells.J Immunol. 1996 Apr 1;156(7):2558-65. J Immunol. 1996. PMID: 8786319
Cited by
-
Extracellular vesicles and endothelial dysfunction in infectious diseases.J Extracell Biol. 2024 Apr 12;3(4):e148. doi: 10.1002/jex2.148. eCollection 2024 Apr. J Extracell Biol. 2024. PMID: 38938849 Free PMC article. Review.
-
Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation.Cell. 2016 May 19;165(5):1106-1119. doi: 10.1016/j.cell.2016.04.015. Epub 2016 May 5. Cell. 2016. PMID: 27156449 Free PMC article.
-
Comparative diet-gut microbiome analysis in Crohn's disease and Hidradenitis suppurativa.Front Microbiol. 2023 Nov 10;14:1289374. doi: 10.3389/fmicb.2023.1289374. eCollection 2023. Front Microbiol. 2023. PMID: 38029085 Free PMC article.
-
Interaction with an endothelial lumen increases neutrophil lifetime and motility in response to P aeruginosa.Blood. 2018 Oct 25;132(17):1818-1828. doi: 10.1182/blood-2018-05-848465. Epub 2018 Aug 24. Blood. 2018. PMID: 30143504 Free PMC article.
-
The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles.Front Microbiol. 2020 Feb 6;11:57. doi: 10.3389/fmicb.2020.00057. eCollection 2020. Front Microbiol. 2020. PMID: 32117106 Free PMC article.
References
-
- Danese S, Dejana E, Fiocchi C (2007) Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol 178: 6017–6022. - PubMed
-
- Harding M, Kubes P (2012) Innate immunity in the vasculature: interactions with pathogenic bacteria. Curr Opin Microbiol 15: 85–91. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689. - PubMed
-
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW (2007) Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res 101: 234–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

