Opa1 is required for proper mitochondrial metabolism in early development
- PMID: 23516612
- PMCID: PMC3597633
- DOI: 10.1371/journal.pone.0059218
Opa1 is required for proper mitochondrial metabolism in early development
Abstract
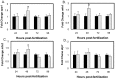
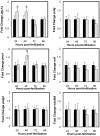
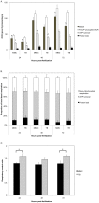
Opa1 catalyzes fusion of inner mitochondrial membranes and formation of the cristae. OPA1 mutations in humans lead to autosomal dominant optic atrophy. OPA1 knockout mice lose viability around embryonic day 9 from unknown reasons, indicating that OPA1 is essential for embryonic development. Zebrafish are an attractive model for studying vertebrate development and have been used for many years to describe developmental events that are difficult or impractical to view in mammalian models. In this study, Opa1 was successfully depleted in zebrafish embryos using antisense morpholinos, which resulted in disrupted mitochondrial morphology. Phenotypically, these embryos exhibited abnormal blood circulation and heart defects, as well as small eyes and small pectoral fin buds. Additionally, startle response was reduced and locomotor activity was impaired. Furthermore, Opa1 depletion caused bioenergetic defects, without impairing mitochondrial efficiency. In response to mitochondrial dysfunction, a transient upregulation of the master regulator of mitochondrial biogenesis, pgc1a, was observed. These results not only reveal a new Opa1-associated phenotype in a vertebrate model system, but also further elucidates the absolute requirement of Opa1 for successful vertebrate development.
Conflict of interest statement
Figures
Similar articles
-
A zebrafish model to study small-fiber neuropathy reveals a potential role for GDAP1.Mitochondrion. 2019 Jul;47:273-281. doi: 10.1016/j.mito.2019.01.002. Epub 2019 Jan 22. Mitochondrion. 2019. PMID: 30677530
-
The defective expression of gtpbp3 related to tRNA modification alters the mitochondrial function and development of zebrafish.Int J Biochem Cell Biol. 2016 Aug;77(Pt A):1-9. doi: 10.1016/j.biocel.2016.05.012. Epub 2016 May 13. Int J Biochem Cell Biol. 2016. PMID: 27184967
-
Mitochondrial dysfunction in an Opa1(Q285STOP) mouse model of dominant optic atrophy results from Opa1 haploinsufficiency.Cell Death Dis. 2016 Jul 28;7(7):e2309. doi: 10.1038/cddis.2016.160. Cell Death Dis. 2016. PMID: 27468686 Free PMC article.
-
Knockdown of fbxl10/kdm2bb rescues chd7 morphant phenotype in a zebrafish model of CHARGE syndrome.Dev Biol. 2013 Oct 1;382(1):57-69. doi: 10.1016/j.ydbio.2013.07.026. Epub 2013 Aug 3. Dev Biol. 2013. PMID: 23920116 Free PMC article.
-
OPA1: How much do we know to approach therapy?Pharmacol Res. 2018 May;131:199-210. doi: 10.1016/j.phrs.2018.02.018. Epub 2018 Feb 15. Pharmacol Res. 2018. PMID: 29454676 Review.
Cited by
-
The cellular and molecular progression of mitochondrial dysfunction induced by 2,4-dinitrophenol in developing zebrafish embryos.Differentiation. 2015 Mar-Apr;89(3-4):51-69. doi: 10.1016/j.diff.2015.01.001. Epub 2015 Mar 12. Differentiation. 2015. PMID: 25771346 Free PMC article.
-
Reduction of the ATPase inhibitory factor 1 (IF1) leads to visual impairment in vertebrates.Cell Death Dis. 2018 Jun 4;9(6):669. doi: 10.1038/s41419-018-0578-x. Cell Death Dis. 2018. PMID: 29867190 Free PMC article.
-
Restoration of Opa1-long isoform inhibits retinal injury-induced neurodegeneration.J Mol Med (Berl). 2016 Mar;94(3):335-46. doi: 10.1007/s00109-015-1359-y. Epub 2015 Nov 4. J Mol Med (Berl). 2016. PMID: 26530815
-
Mitochondrial Dynamics Drive Muscle Stem Cell Progression from Quiescence to Myogenic Differentiation.Cells. 2024 Oct 26;13(21):1773. doi: 10.3390/cells13211773. Cells. 2024. PMID: 39513880 Free PMC article. Review.
-
SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury.Aging (Albany NY). 2020 Jul 28;12(16):16224-16237. doi: 10.18632/aging.103644. Epub 2020 Jul 28. Aging (Albany NY). 2020. PMID: 32721927 Free PMC article.
References
-
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, et al. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189. - PubMed
-
- Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, et al. (2007) OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ 14: 682–692. - PubMed
-
- Olichon A, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, et al. (2007) Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. J Cell Physiol 211: 423–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

