Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor-β signaling protein Smad2
- PMID: 23516280
- PMCID: PMC3684282
- DOI: 10.1523/JNEUROSCI.5321-12.2013
Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor-β signaling protein Smad2
Abstract
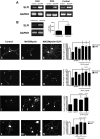
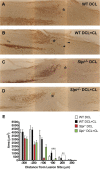
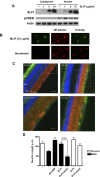
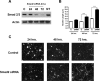
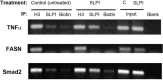
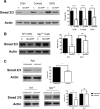
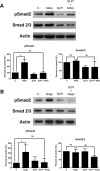
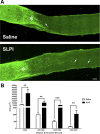
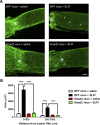
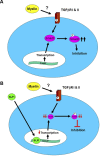
After CNS injury, axonal regeneration is limited by myelin-associated inhibitors; however, this can be overcome through elevation of intracellular cyclic AMP (cAMP), as occurs with conditioning lesions of the sciatic nerve. This study reports that expression of secretory leukocyte protease inhibitor (SLPI) is strongly upregulated in response to elevation of cAMP. We also show that SLPI can overcome inhibition by CNS myelin and significantly enhance regeneration of transected retinal ganglion cell axons in rats. Furthermore, regeneration of dorsal column axons does not occur after a conditioning lesion in SLPI null mutant mice, indicating that expression of SLPI is required for the conditioning lesion effect. Mechanistically, we demonstrate that SLPI localizes to the nuclei of neurons, binds to the Smad2 promoter, and reduces levels of Smad2 protein. Adenoviral overexpression of Smad2 also blocked SLPI-induced axonal regeneration. SLPI and Smad2 may therefore represent new targets for therapeutic intervention in CNS injury.
Figures
Similar articles
-
Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo.J Neurosci. 2009 Jul 29;29(30):9545-52. doi: 10.1523/JNEUROSCI.1175-09.2009. J Neurosci. 2009. PMID: 19641117 Free PMC article.
-
Schwann cell-derived factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits axon growth through CNS myelin in vivo and in vitro.Brain. 2006 Jun;129(Pt 6):1517-33. doi: 10.1093/brain/awl080. Epub 2006 Apr 13. Brain. 2006. PMID: 16613894
-
Secretory Leukocyte Protease Inhibitor (SLPI): Emerging Roles in CNS Trauma and Repair.Neuroscientist. 2015 Dec;21(6):630-6. doi: 10.1177/1073858414546000. Epub 2014 Aug 12. Neuroscientist. 2015. PMID: 25118190 Review.
-
Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state.J Neurosci. 2004 Feb 18;24(7):1646-51. doi: 10.1523/JNEUROSCI.5119-03.2004. J Neurosci. 2004. PMID: 14973241 Free PMC article.
-
Return of function after CNS axon regeneration: Lessons from injury-responsive intrinsically photosensitive and alpha retinal ganglion cells.Prog Retin Eye Res. 2019 Jul;71:57-67. doi: 10.1016/j.preteyeres.2018.11.006. Epub 2018 Nov 17. Prog Retin Eye Res. 2019. PMID: 30458239 Review.
Cited by
-
The Dyslexia-susceptibility Protein KIAA0319 Inhibits Axon Growth Through Smad2 Signaling.Cereb Cortex. 2017 Mar 1;27(3):1732-1747. doi: 10.1093/cercor/bhx023. Cereb Cortex. 2017. PMID: 28334068 Free PMC article.
-
Looking downstream: the role of cyclic AMP-regulated genes in axonal regeneration.Front Mol Neurosci. 2015 Jun 18;8:26. doi: 10.3389/fnmol.2015.00026. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26150769 Free PMC article. Review.
-
Fasudil, a Rho-Associated Protein Kinase Inhibitor, Attenuates Traumatic Retinal Nerve Injury in Rabbits.J Mol Neurosci. 2016 Jan;58(1):74-82. doi: 10.1007/s12031-015-0691-6. Epub 2015 Dec 3. J Mol Neurosci. 2016. PMID: 26635024
-
Flipping the transcriptional switch from myelin inhibition to axon growth in the CNS.Front Mol Neurosci. 2015 Jul 17;8:34. doi: 10.3389/fnmol.2015.00034. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26236189 Free PMC article.
-
Opposing Effects of Growth and Differentiation Factors in Cell-Fate Specification.Curr Biol. 2019 Jun 17;29(12):1963-1975.e5. doi: 10.1016/j.cub.2019.05.011. Epub 2019 May 30. Curr Biol. 2019. PMID: 31155355 Free PMC article.
References
-
- Abdollah S, Macías-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. - PubMed
-
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. - PubMed
-
- Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. TGF-beta1 and TGF-beta2 expression after traumatic human spinal cord injury. Spinal Cord. 2008;46:364–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
