A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses
- PMID: 23515440
- PMCID: PMC4028127
- DOI: 10.1136/annrheumdis-2012-202716
A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses
Abstract
Background: Persistent fibroblast activation initiated by transforming growth factor β (TGF-β) is a fundamental event in the pathogenesis of systemic sclerosis, and its pharmacological inhibition represents a potential therapeutic strategy. The nuclear receptor, peroxisome proliferator-activated receptor γ (PPAR-γ), exerts potent fibrotic activity. The synthetic oleanane triterpenoid, 2-cyano-3,12-dioxo-olean-1,9-dien-28-oic acid (CDDO), is a PPAR-γ agonist with potential effects on TGF-β signalling and dermal fibrosis.
Objective: To examine the modulation of fibrogenesis by CDDO in explanted fibroblasts, skin organ cultures and murine models of scleroderma.
Material and methods: The effects of CDDO on experimental fibrosis induced by bleomycin injection or by overexpression of constitutively active type I TGF-β receptor (TgfbR1ca) were evaluated. Modulation of fibrotic gene expression was examined in human skin organ cultures. To delineate the mechanisms underlying the antifibrotic effects of CDDO, explanted skin fibroblasts cultured in two-dimensional monolayers or in three-dimensional full-thickness human skin equivalents were studied.
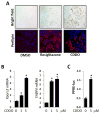
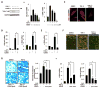
Results: CDDO significantly ameliorated dermal fibrosis in two complementary mouse models of scleroderma, as well as in human skin organ cultures and in three-dimensional human skin equivalents. In two-dimensional monolayer cultures of explanted normal fibroblasts, CDDO abrogated fibrogenic responses induced by TGF-β. These CDDO effects occurred via disruption of Smad-dependent transcription and were associated with inhibition of Akt activation. In scleroderma fibroblasts, CDDO attenuated the elevated synthesis of collagen. Remarkably, the in vitro antifibrotic effects of CDDO were independent of PPAR-γ.
Conclusions: The PPAR-γ agonist triterpenoid CDDO attenuates fibrogenesis by antagonistically targeting canonical TGF-β/Smad and Akt signalling in a PPAR-γ-independent manner. These findings identify this synthetic triterpenoid as a potential new therapy for the control of fibrosis.
Keywords: CDDO; PPAR-γ; TGF-β; fibroblast; fibrosis; murine scleroderma; triterpenoid.
Conflict of interest statement
Figures



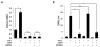
Similar articles
-
The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy.Arthritis Res Ther. 2012 Oct 23;14(5):R229. doi: 10.1186/ar4070. Arthritis Res Ther. 2012. PMID: 23092446 Free PMC article.
-
Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma.Arthritis Rheum. 2004 Apr;50(4):1305-18. doi: 10.1002/art.20104. Arthritis Rheum. 2004. PMID: 15077315
-
Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts.Am J Respir Cell Mol Biol. 2009 Dec;41(6):722-30. doi: 10.1165/rcmb.2009-0006OC. Epub 2009 Mar 13. Am J Respir Cell Mol Biol. 2009. PMID: 19286977 Free PMC article.
-
Pathogenesis of scleroderma. Collagen.Rheum Dis Clin North Am. 1996 Nov;22(4):647-74. doi: 10.1016/s0889-857x(05)70294-5. Rheum Dis Clin North Am. 1996. PMID: 8923589 Review.
-
Signaling in fibrosis: targeting the TGF beta, endothelin-1 and CCN2 axis in scleroderma.Front Biosci (Elite Ed). 2009 Jun 1;1(1):115-22. doi: 10.2741/E12. Front Biosci (Elite Ed). 2009. PMID: 19482630 Review.
Cited by
-
Therapeutic potential of PPARγ natural agonists in liver diseases.J Cell Mol Med. 2020 Mar;24(5):2736-2748. doi: 10.1111/jcmm.15028. Epub 2020 Feb 7. J Cell Mol Med. 2020. PMID: 32031298 Free PMC article. Review.
-
Genetics of systemic sclerosis.Semin Immunopathol. 2015 Sep;37(5):443-51. doi: 10.1007/s00281-015-0499-z. Epub 2015 Jun 2. Semin Immunopathol. 2015. PMID: 26032405 Review.
-
The Histone Deacetylase Sirtuin 1 Is Reduced in Systemic Sclerosis and Abrogates Fibrotic Responses by Targeting Transforming Growth Factor β Signaling.Arthritis Rheumatol. 2015 May;67(5):1323-34. doi: 10.1002/art.39061. Arthritis Rheumatol. 2015. PMID: 25707573 Free PMC article.
-
Shared and distinct mechanisms of fibrosis.Nat Rev Rheumatol. 2019 Dec;15(12):705-730. doi: 10.1038/s41584-019-0322-7. Epub 2019 Nov 11. Nat Rev Rheumatol. 2019. PMID: 31712723 Review.
-
Fibrosis--a lethal component of systemic sclerosis.Nat Rev Rheumatol. 2014 Jul;10(7):390-402. doi: 10.1038/nrrheum.2014.53. Epub 2014 Apr 22. Nat Rev Rheumatol. 2014. PMID: 24752182 Review.
References
-
- Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152(3):159–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
