Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses
- PMID: 23514880
- PMCID: PMC3648176
- DOI: 10.1128/JVI.00120-13
Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses
Abstract
Influenza viruses often evade host immunity via antigenic drift and shift despite previous influenza virus infection and/or vaccination. Vaccines that match circulating virus strains are needed for optimal protection. Development of a universal influenza virus vaccine providing broadly cross-protective immunity will be of great importance. The nucleoprotein (NP) of influenza A virus is highly conserved among all strains of influenza A viruses and has been explored as an antigen for developing a universal influenza virus vaccine. In this work, we generated a recombinant parainfluenza virus 5 (PIV5) containing NP from H5N1 (A/Vietnam/1203/2004), a highly pathogenic avian influenza (HPAI) virus, between HN and L (PIV5-NP-HN/L) and tested its efficacy. PIV5-NP-HN/L induced humoral and T cell responses in mice. A single inoculation of PIV5-NP-HN/L provided complete protection against lethal heterosubtypic H1N1 challenge and 50% protection against lethal H5N1 HPAI virus challenge. To improve efficacy, NP was inserted into different locations within the PIV5 genome. Recombinant PIV5 containing NP between F and SH (PIV5-NP-F/SH) or between SH and HN (PIV5-NP-SH/HN) provided better protection against H5N1 HPAI virus challenge than did PIV5-NP-HN/L. These results suggest that PIV5 expressing NP from H5N1 has the potential to be utilized as a universal influenza virus vaccine.
Figures


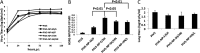
Similar articles
-
Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge.J Virol. 2013 Jan;87(1):354-62. doi: 10.1128/JVI.02321-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077314 Free PMC article.
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
A single dose of DNA vaccine based on conserved H5N1 subtype proteins provides protection against lethal H5N1 challenge in mice pre-exposed to H1N1 influenza virus.Virol J. 2010 Aug 21;7:197. doi: 10.1186/1743-422X-7-197. Virol J. 2010. PMID: 20727202 Free PMC article.
-
Progress on adenovirus-vectored universal influenza vaccines.Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review.
-
Development of universal influenza vaccines based on influenza virus M and NP genes.Infection. 2014 Apr;42(2):251-62. doi: 10.1007/s15010-013-0546-4. Epub 2013 Nov 1. Infection. 2014. PMID: 24178189 Review.
Cited by
-
Parainfluenza Virus 5 Priming Followed by SIV/HIV Virus-Like-Particle Boosting Induces Potent and Durable Immune Responses in Nonhuman Primates.Front Immunol. 2021 Feb 25;12:623996. doi: 10.3389/fimmu.2021.623996. eCollection 2021. Front Immunol. 2021. PMID: 33717130 Free PMC article.
-
How sticky should a virus be? The impact of virus binding and release on transmission fitness using influenza as an example.J R Soc Interface. 2014 Jan 15;11(92):20131083. doi: 10.1098/rsif.2013.1083. Print 2014 Mar 6. J R Soc Interface. 2014. PMID: 24430126 Free PMC article.
-
Parainfluenza Virus 5 Expressing Wild-Type or Prefusion Respiratory Syncytial Virus (RSV) Fusion Protein Protects Mice and Cotton Rats from RSV Challenge.J Virol. 2017 Sep 12;91(19):e00560-17. doi: 10.1128/JVI.00560-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747496 Free PMC article.
-
A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5).Vaccine. 2014 May 23;32(25):3050-7. doi: 10.1016/j.vaccine.2014.03.049. Epub 2014 Apr 8. Vaccine. 2014. PMID: 24717150 Free PMC article.
-
Centralized Consensus Hemagglutinin Genes Induce Protective Immunity against H1, H3 and H5 Influenza Viruses.PLoS One. 2015 Oct 15;10(10):e0140702. doi: 10.1371/journal.pone.0140702. eCollection 2015. PLoS One. 2015. PMID: 26469190 Free PMC article.
References
-
- Wright PFW. 2001. Orthomyxoviruses, p 1533–1579 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA
-
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, Turmelle AS, Moran D, Rogers S, Shi M, Tao Y, Weil MR, Tang K, Rowe LA, Sammons S, Xu X, Frace M, Lindblade KA, Cox NJ, Anderson LJ, Rupprecht CE, Donis RO. 2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U. S. A. 109:4269–4274 - PMC - PubMed
-
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. 2005. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 54(RR8):1–40 - PubMed
-
- Weekly Epidemiological Record 2003. Influenza fact sheet. Wkly. Epidemiol. Rec. 78:77–80 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

