Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes
- PMID: 23514733
- PMCID: PMC3714510
- DOI: 10.2337/dc12-1835
Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes
Abstract
Objective: Inflammation is associated with pancreatic β-cell apoptosis and reduced insulin sensitivity. Literature suggests that interleukin (IL)-1β may contribute to the pathogenesis of type 2 diabetes mellitus (T2DM). This study aimed to determine the efficacy, safety, and tolerability of LY2189102, a neutralizing IL-1β antibody, in T2DM patients.
Research design and methods: Phase II, randomized, double-blind, parallel, placebo-controlled study of subcutaneous LY2189102 (0.6, 18, and 180 mg) administered weekly for 12 weeks in T2DM patients on diet and exercise, with or without approved antidiabetic medications.
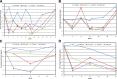
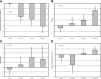
Results: LY2189102 reduced HbA1c at 12 weeks (adjusted mean differences versus placebo: -0.27, -0.38 and -0.25% for 0.6, 18 and 180 mg doses, respectively), and fasting glucose at multiple time points compared with placebo. LY2189102 also reduced postprandial glycemia, and inflammatory biomarkers, including hs-CRP and IL-6. LY2189102 was generally well tolerated.
Conclusions: Weekly subcutaneous LY2189102 for 12 weeks was well tolerated, modestly reduced HbA1c and fasting glucose, and demonstrated significant anti-inflammatory effects in T2DM patients. Neutralizing IL-1β holds promise as a convenient adjuvant treatment for T2DM.
Figures
Similar articles
-
Impact of interleukin-1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial.Diabetes Metab. 2013 Dec;39(6):524-31. doi: 10.1016/j.diabet.2013.07.003. Epub 2013 Sep 25. Diabetes Metab. 2013. PMID: 24075453 Clinical Trial.
-
Population pharmacokinetic modeling of LY2189102 after multiple intravenous and subcutaneous administrations.AAPS J. 2014 Sep;16(5):1009-17. doi: 10.1208/s12248-014-9623-6. Epub 2014 Jun 11. AAPS J. 2014. PMID: 24912797 Free PMC article.
-
Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension.Diabetes Obes Metab. 2014 May;16(5):418-25. doi: 10.1111/dom.12235. Epub 2013 Dec 10. Diabetes Obes Metab. 2014. PMID: 24205974 Clinical Trial.
-
Safety and Efficacy of Canakinumab for the Prevention and Control of Type 2 Diabetes Mellitus and Its Complications: A Systematic Review.Cureus. 2024 Aug 17;16(8):e67065. doi: 10.7759/cureus.67065. eCollection 2024 Aug. Cureus. 2024. PMID: 39286685 Free PMC article. Review.
-
Salsalate: a pleotropic anti-inflammatory drug in the treatment of diabetes, obesity, and metabolic diseases.Inflammopharmacology. 2023 Dec;31(6):2781-2797. doi: 10.1007/s10787-023-01242-9. Epub 2023 Sep 27. Inflammopharmacology. 2023. PMID: 37758933 Review.
Cited by
-
Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity?Adipocyte. 2015 Jan 7;4(2):149-52. doi: 10.4161/21623945.2014.979661. eCollection 2015 Apr-Jun. Adipocyte. 2015. PMID: 26167419 Free PMC article.
-
A novel fast-slow model of diabetes progression: Insights into mechanisms of response to the interventions in the Diabetes Prevention Program.PLoS One. 2019 Oct 10;14(10):e0222833. doi: 10.1371/journal.pone.0222833. eCollection 2019. PLoS One. 2019. PMID: 31600232 Free PMC article.
-
Fructose Induces Insulin Resistance of Gestational Diabetes Mellitus in Mice via the NLRP3 Inflammasome Pathway.Front Nutr. 2022 Apr 12;9:839174. doi: 10.3389/fnut.2022.839174. eCollection 2022. Front Nutr. 2022. PMID: 35495917 Free PMC article.
-
The role of interleukin-1 in general pathology.Inflamm Regen. 2019 Jun 6;39:12. doi: 10.1186/s41232-019-0101-5. eCollection 2019. Inflamm Regen. 2019. PMID: 31182982 Free PMC article. Review.
-
Diabetes and Sepsis: Risk, Recurrence, and Ruination.Front Endocrinol (Lausanne). 2017 Oct 30;8:271. doi: 10.3389/fendo.2017.00271. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29163354 Free PMC article. Review.
References
-
- Cerasi E, Luft R. Insulin response to glucose infusion in diabetic and non-diabetic monozygotic twin pairs. Genetic control of insulin response? Acta Endocrinol (Copenh) 1967;55:330–345 - PubMed
-
- DeFronzo RA. Lilly lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 - PubMed
-
- Hellerström C. The life story of the pancreatic B cell. Diabetologia 1984;26:393–400 - PubMed
-
- Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 2004;47:581–589 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

