Regulation of nonsense-mediated mRNA decay: implications for physiology and disease
- PMID: 23500037
- PMCID: PMC3660545
- DOI: 10.1016/j.bbagrm.2013.03.002
Regulation of nonsense-mediated mRNA decay: implications for physiology and disease
Abstract
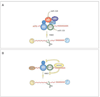
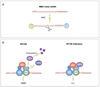
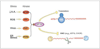
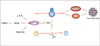
Nonsense-mediated mRNA decay (NMD) is an mRNA quality control mechanism that destabilizes aberrant mRNAs harboring premature termination (nonsense) codons (PTCs). Recent studies have shown that NMD also targets mRNAs transcribed from a large subset of wild-type genes. This raises the possibility that NMD itself is under regulatory control. Indeed, several recent studies have shown that NMD activity is modulated in specific cell types and that key components of the NMD pathway are regulated by several pathways, including microRNA circuits and NMD itself. Cellular stress also modulates the magnitude of NMD by mechanisms that are beginning to be understood. Here, we review the evidence that NMD is regulated and discuss the physiological role for this regulation. We propose that the efficiency of NMD is altered in some cellular contexts to regulate normal biological events. In disease states-such as in cancer-NMD is disturbed by intrinsic and extrinsic factors, resulting in altered levels of crucial NMD-targeted mRNAs that lead to downstream pathological consequences. This article is part of a Special Issue entitled: RNA Decay mechanisms.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Mechanism and regulation of the nonsense-mediated decay pathway.Nucleic Acids Res. 2016 Feb 29;44(4):1483-95. doi: 10.1093/nar/gkw010. Epub 2016 Jan 14. Nucleic Acids Res. 2016. PMID: 26773057 Free PMC article. Review.
-
Nonsense-mediated mRNA decay at the crossroads of many cellular pathways.BMB Rep. 2017 Apr;50(4):175-185. doi: 10.5483/bmbrep.2017.50.4.015. BMB Rep. 2017. PMID: 28115040 Free PMC article. Review.
-
Unspliced precursors of NMD-sensitive β-globin transcripts exhibit decreased steady-state levels in erythroid cells.PLoS One. 2012;7(6):e38505. doi: 10.1371/journal.pone.0038505. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675570 Free PMC article.
-
Physiological and pathophysiological role of nonsense-mediated mRNA decay.Pflugers Arch. 2016 Jun;468(6):1013-28. doi: 10.1007/s00424-016-1826-5. Epub 2016 Apr 30. Pflugers Arch. 2016. PMID: 27138169 Review.
-
Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes.Nat Rev Mol Cell Biol. 2015 Nov;16(11):665-77. doi: 10.1038/nrm4063. Epub 2015 Sep 23. Nat Rev Mol Cell Biol. 2015. PMID: 26397022 Review.
Cited by
-
A novel human-specific splice isoform alters the critical C-terminus of Survival Motor Neuron protein.Sci Rep. 2016 Aug 2;6:30778. doi: 10.1038/srep30778. Sci Rep. 2016. PMID: 27481219 Free PMC article.
-
Ribosome Recycling by ABCE1 Links Lysosomal Function and Iron Homeostasis to 3' UTR-Directed Regulation and Nonsense-Mediated Decay.Cell Rep. 2020 Jul 14;32(2):107895. doi: 10.1016/j.celrep.2020.107895. Cell Rep. 2020. PMID: 32668236 Free PMC article.
-
Novel splice-affecting variants in CYP27A1 gene in two Chilean patients with Cerebrotendinous Xanthomatosis.Genet Mol Biol. 2015 Mar;38(1):30-6. doi: 10.1590/S1415-475738120140087. Epub 2014 Mar 17. Genet Mol Biol. 2015. PMID: 25983621 Free PMC article.
-
Attenuation of nonsense-mediated mRNA decay facilitates the response to chemotherapeutics.Nat Commun. 2015 Mar 26;6:6632. doi: 10.1038/ncomms7632. Nat Commun. 2015. PMID: 25808464 Free PMC article.
-
Coupling mRNA synthesis and decay.Mol Cell Biol. 2014 Nov 15;34(22):4078-87. doi: 10.1128/MCB.00535-14. Epub 2014 Aug 25. Mol Cell Biol. 2014. PMID: 25154419 Free PMC article. Review.
References
-
- Chang Y-F, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annual Review of Biochemistry. 2007;76:51–74. - PubMed
-
- Frischmeyer PA, Dietz HC. Nonsense-mediated mRNA decay in health and disease. Human Molecular Genetics. 1999;8:1893–1900. - PubMed
-
- Holbrook JA, Neu-Yilik G, Hentze MW, Kulozik AE. Nonsense-mediated decay approaches the clinic. Nat Genet. 2004;36:801–808. - PubMed
-
- Rebbapragada I, Lykke-Andersen J. Execution of nonsense-mediated mRNA decay: what defines a substrate? Current Opinion in Cell Biology. 2009;21:394–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

