Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice
- PMID: 23497784
- PMCID: PMC3640742
- DOI: 10.1016/j.atherosclerosis.2013.02.023
Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice
Abstract
Objective: There is an emerging widespread interest in the role of damage-associated molecular pattern molecules (DAMP) S100A8, S100A9 and S100A12 in cardiovascular and other diseases. In this study we tested the efficacy of ABR-215757, a S100 protein binding immuno-modulatory compound to stabilize atherosclerosis in transgenic ApoE null mice that express the human pro-inflammatory S100A12 protein within the smooth muscle cell (SM22α-S100A12).
Methods: Twelve-week old S100A12 transgenic/ApoE(-/-) and WT/ApoE(-/-) mice were treated with ABR-21575 for 5 weeks and were analyzed 4 month later.
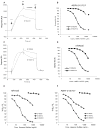
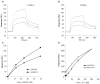
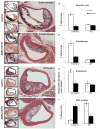
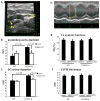
Results: Surface plasmon resonance analysis demonstrated that S100A12 interacts with ABR-215757 in a zinc dependent manner in vitro. In vivo, ABR-215757 administration reduced features of advanced plaque morphology resulting in smaller necrotic cores, diminished intimal and medial vascular calcification, and reduced amount of infiltrating inflammatory cells. ABR-215757 normalized aortic expression of RAGE protein and normalized experimentally-induced delayed hypersensitivity. The effect of ABR-215757 was more prominent in ApoE(-/-) mice expressing S100A12 than in ApoE(-/-) animals lacking expression of human S100A12 protein.
Conclusion: Our data suggest that S100A12 is important for progression of atherosclerosis and can be targeted by the small molecule ABR-215757. The specific binding of quinoline-3-carboxamides to S100A12 attenuates S100A12-mediated features of accelerated murine atherosclerosis.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2496-507. doi: 10.1161/ATVBAHA.115.302072. Epub 2015 Oct 29. Arterioscler Thromb Vasc Biol. 2015. PMID: 26515415 Free PMC article. Review.
-
S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program.Arterioscler Thromb Vasc Biol. 2011 Feb;31(2):337-44. doi: 10.1161/ATVBAHA.110.217745. Epub 2010 Oct 21. Arterioscler Thromb Vasc Biol. 2011. PMID: 20966394 Free PMC article.
-
IL-22 is induced by S100/calgranulin and impairs cholesterol efflux in macrophages by downregulating ABCG1.J Lipid Res. 2014 Mar;55(3):443-54. doi: 10.1194/jlr.M044305. Epub 2013 Dec 23. J Lipid Res. 2014. PMID: 24367046 Free PMC article.
-
S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner.Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1399-411. doi: 10.1161/ATVBAHA.114.303508. Epub 2014 May 22. Arterioscler Thromb Vasc Biol. 2014. PMID: 24855059 Free PMC article.
-
S100 proteins in atherosclerosis.Clin Chim Acta. 2020 Mar;502:293-304. doi: 10.1016/j.cca.2019.11.019. Epub 2019 Nov 30. Clin Chim Acta. 2020. PMID: 31794767 Review.
Cited by
-
S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease.Mediators Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. Epub 2013 Dec 22. Mediators Inflamm. 2013. PMID: 24453429 Free PMC article. Review.
-
Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications.Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):205-217. doi: 10.1161/ATVBAHA.116.306258. Epub 2016 Dec 22. Arterioscler Thromb Vasc Biol. 2017. PMID: 28062508 Free PMC article. Review.
-
Increased serum S100A12 levels are associated with higher risk of acute heart failure in patients with type 2 diabetes.ESC Heart Fail. 2022 Dec;9(6):3909-3919. doi: 10.1002/ehf2.14036. Epub 2022 Aug 11. ESC Heart Fail. 2022. PMID: 36637406 Free PMC article.
-
Atorvastatin Reduces Circulating S100A12 Levels in Patients with Carotid Atherosclerotic Plaques - A Link with Plaque Inflammation.J Atheroscler Thromb. 2022 May 1;29(5):775-784. doi: 10.5551/jat.61630. Epub 2021 May 1. J Atheroscler Thromb. 2022. PMID: 33952812 Free PMC article. Clinical Trial.
-
An open-label study to evaluate biomarkers and safety in systemic sclerosis patients treated with paquinimod.Arthritis Res Ther. 2021 Jul 31;23(1):204. doi: 10.1186/s13075-021-02573-0. Arthritis Res Ther. 2021. PMID: 34330322 Free PMC article.
References
-
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. - PubMed
-
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. - PubMed
-
- Rosenberg S, Elashoff MR, Beineke P, Daniels SE, Wingrove JA, Tingley WG, Sager PT, Sehnert AJ, Yau M, Kraus WE, Newby LK, Schwartz RS, Voros S, Ellis SG, Tahirkheli N, Waksman R, McPherson J, Lansky A, Winn ME, Schork NJ, Topol EJ. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010;153:425–434. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

