Zap70 inhibits Syk-mediated osteoclast function
- PMID: 23494777
- PMCID: PMC3977572
- DOI: 10.1002/jcb.24531
Zap70 inhibits Syk-mediated osteoclast function
Abstract
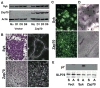
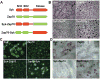
The αvβ3 integrin stimulates the resorptive capacity of the differentiated osteoclast (OC) by organizing its cytoskeleton via the tyrosine kinase, Syk. Thus, Syk-deficient OCs fails to spread or form actin rings, in vitro and in vivo. The Syk family of tyrosine kinases consists of Syk itself and Zap70 which are expressed by different cell types. Because of their structural similarity, and its compensatory properties in other cells, we asked if Zap70 can substitute for absence of Syk in OCs. While expression of Syk, as expected, normalizes the cytoskeletal abnormalities of Syk(-/-) OCs, Zap70 fails do so. In keeping with this observation, Syk, but not Zap70, rescues αvβ3 integrin-induced SLP76 phosphorylation in Syk(-/-) OCs. Furthermore the kinase sequence of Syk partially rescues the Syk(-/-) phenotype but full normalization also requires its SH2 domains. Surprisingly, expression of Zap70 inhibits WT OC spreading, actin ring formation and bone resorptive activity, but not differentiation. In keeping with arrested cytoskeletal organization, Zap70 blocks integrin-activated endogenous Syk and Vav3, SLP76 phosphorylation. Such inhibition requires Zap70 kinase activity, as it is abolished by mutation of the Zap70 kinase domain. Thus, while the kinase domain of Syk is uniquely required for OC function that of Zap70 inhibits it.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare they have no financial conflicts of interest.
Figures

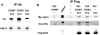

Similar articles
-
SLP-76 couples Syk to the osteoclast cytoskeleton.J Immunol. 2009 Aug 1;183(3):1804-12. doi: 10.4049/jimmunol.0804206. Epub 2009 Jul 10. J Immunol. 2009. PMID: 19592646 Free PMC article.
-
An osteoclastic protein-tyrosine phosphatase regulates the β3-integrin, syk, and shp1 signaling through respective src-dependent phosphorylation in osteoclasts.Am J Physiol Cell Physiol. 2012 Jun 1;302(11):C1676-86. doi: 10.1152/ajpcell.00042.2012. Epub 2012 Mar 28. Am J Physiol Cell Physiol. 2012. PMID: 22460711
-
Syk tyrosine 317 negatively regulates osteoclast function via the ubiquitin-protein isopeptide ligase activity of Cbl.J Biol Chem. 2009 Jul 10;284(28):18833-9. doi: 10.1074/jbc.M109.012385. Epub 2009 May 6. J Biol Chem. 2009. PMID: 19419964 Free PMC article.
-
The osteoclast and its unique cytoskeleton.Ann N Y Acad Sci. 2011 Dec;1240:14-7. doi: 10.1111/j.1749-6632.2011.06283.x. Ann N Y Acad Sci. 2011. PMID: 22172034 Review.
-
Role of spleen tyrosine kinase in the pathogenesis of chronic lymphocytic leukemia.Leuk Lymphoma. 2014 Dec;55(12):2699-705. doi: 10.3109/10428194.2014.891026. Epub 2014 Mar 18. Leuk Lymphoma. 2014. PMID: 24547708 Review.
Cited by
-
Vinculin regulates osteoclast function.J Biol Chem. 2014 May 9;289(19):13554-64. doi: 10.1074/jbc.M114.550731. Epub 2014 Mar 27. J Biol Chem. 2014. PMID: 24675074 Free PMC article.
-
γδTCR recruits the Syk/PI3K axis to drive proinflammatory differentiation program.J Clin Invest. 2018 Jan 2;128(1):415-426. doi: 10.1172/JCI95837. Epub 2017 Dec 4. J Clin Invest. 2018. PMID: 29202478 Free PMC article.
-
Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli.J Cell Mol Med. 2019 May;23(5):3077-3087. doi: 10.1111/jcmm.14277. Epub 2019 Mar 20. J Cell Mol Med. 2019. PMID: 30892789 Free PMC article. Review.
-
Hematopoietic or Osteoclast-Specific Deletion of Syk Leads to Increased Bone Mass in Experimental Mice.Front Immunol. 2019 Apr 30;10:937. doi: 10.3389/fimmu.2019.00937. eCollection 2019. Front Immunol. 2019. PMID: 31134061 Free PMC article.
-
Osteoclasts-Key Players in Skeletal Health and Disease.Microbiol Spectr. 2016 Jun;4(3):10.1128/microbiolspec.MCHD-0011-2015. doi: 10.1128/microbiolspec.MCHD-0011-2015. Microbiol Spectr. 2016. PMID: 27337470 Free PMC article. Review.
References
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
-
- Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. - PubMed
-
- Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype. J Cell Biochem. 2003;90:871–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR032788/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- AR046523/AR/NIAMS NIH HHS/United States
- HL74138/HL/NHLBI NIH HHS/United States
- HL53325/HL/NHLBI NIH HHS/United States
- AR057037/AR/NIAMS NIH HHS/United States
- R01 HL074138/HL/NHLBI NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- R37 HL053325/HL/NHLBI NIH HHS/United States
- R01 HL053325/HL/NHLBI NIH HHS/United States
- R01 AR057037/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

