Impact of enzymatic and alkaline hydrolysis on CBD concentration in urine
- PMID: 23494274
- PMCID: PMC3703206
- DOI: 10.1007/s00216-013-6837-x
Impact of enzymatic and alkaline hydrolysis on CBD concentration in urine
Abstract
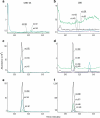
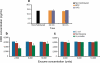
A sensitive and specific analytical method for cannabidiol (CBD) in urine was needed to define urinary CBD pharmacokinetics after controlled CBD administration, and to confirm compliance with CBD medications including Sativex-a cannabis plant extract containing 1:1 ∆(9)-tetrahydrocannabinol (THC) and CBD. Non-psychoactive CBD has a wide range of therapeutic applications and may also influence psychotropic smoked cannabis effects. Few methods exist for the quantification of CBD excretion in urine, and no data are available for phase II metabolism of CBD to CBD-glucuronide or CBD-sulfate. We optimized the hydrolysis of CBD-glucuronide and/or -sulfate, and developed and validated a GC-MS method for urinary CBD quantification. Solid-phase extraction isolated and concentrated analytes prior to GC-MS. Method validation included overnight hydrolysis (16 h) at 37 °C with 2,500 units β-glucuronidase from Red Abalone. Calibration curves were fit by linear least squares regression with 1/x (2) weighting with linear ranges (r(2) > 0.990) of 2.5-100 ng/mL for non-hydrolyzed CBD and 2.5-500 ng/mL for enzyme-hydrolyzed CBD. Bias was 88.7-105.3 %, imprecision 1.4-6.4 % CV and extraction efficiency 82.5-92.7 % (no hydrolysis) and 34.3-47.0 % (enzyme hydrolysis). Enzyme-hydrolyzed urine specimens exhibited more than a 250-fold CBD concentration increase compared to alkaline and non-hydrolyzed specimens. This method can be applied for urinary CBD quantification and further pharmacokinetics characterization following controlled CBD administration.
Figures
Similar articles
-
Optimization of recombinant β-glucuronidase hydrolysis and quantification of eight urinary cannabinoids and metabolites by liquid chromatography tandem mass spectrometry.Drug Test Anal. 2018 Mar;10(3):518-529. doi: 10.1002/dta.2230. Epub 2017 Aug 16. Drug Test Anal. 2018. PMID: 28815938
-
Simultaneous GC-EI-MS determination of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-Delta9-tetrahydrocannabinol in human urine following tandem enzyme-alkaline hydrolysis.J Anal Toxicol. 2007 Oct;31(8):477-85. doi: 10.1093/jat/31.8.477. J Anal Toxicol. 2007. PMID: 17988462 Free PMC article. Clinical Trial.
-
THC and CBD concentrations in blood, oral fluid and urine following a single and repeated administration of "light cannabis".Clin Chem Lab Med. 2020 Apr 28;58(5):682-689. doi: 10.1515/cclm-2019-0119. Clin Chem Lab Med. 2020. PMID: 30956228
-
Preliminary data on the potential for unintentional antidoping rule violations by permitted cannabidiol (CBD) use.Drug Test Anal. 2021 Mar;13(3):539-549. doi: 10.1002/dta.2959. Epub 2020 Dec 14. Drug Test Anal. 2021. PMID: 33125823
-
Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry.J Chromatogr A. 2010 Feb 26;1217(9):1513-21. doi: 10.1016/j.chroma.2009.12.053. Epub 2010 Jan 4. J Chromatogr A. 2010. PMID: 20083251 Free PMC article.
Cited by
-
Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans.J Addict Med. 2015 May-Jun;9(3):204-10. doi: 10.1097/ADM.0000000000000118. J Addict Med. 2015. PMID: 25748562 Free PMC article. Clinical Trial.
-
Correlation of Nabiximols Dose to Steady-State Concentrations of Cannabinoids in Urine Samples from Patients with Multiple Sclerosis.J Clin Med. 2022 Jun 27;11(13):3717. doi: 10.3390/jcm11133717. J Clin Med. 2022. PMID: 35807001 Free PMC article.
-
A urinary test procedure for identification of cannabidiol in patients undergoing medical therapy with marijuana.J Pain Res. 2016 Feb 12;9:81-5. doi: 10.2147/JPR.S96856. eCollection 2016. J Pain Res. 2016. PMID: 26929665 Free PMC article.
-
Cannabinoid concentrations in confiscated cannabis samples and in whole blood and urine after smoking CBD-rich cannabis as a "tobacco substitute".Int J Legal Med. 2019 May;133(3):821-832. doi: 10.1007/s00414-018-01994-y. Epub 2019 Jan 5. Int J Legal Med. 2019. PMID: 30612324
-
A Conversion of Oral Cannabidiol to Delta9-Tetrahydrocannabinol Seems Not to Occur in Humans.Cannabis Cannabinoid Res. 2017 May 1;2(1):81-86. doi: 10.1089/can.2017.0009. eCollection 2017. Cannabis Cannabinoid Res. 2017. PMID: 28861507 Free PMC article.
References
-
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. - PubMed
-
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schroder N, Nardi AE, Martin-Santos R, Hallak JE, Zuardi AW, Crippa JA. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. - PMC - PubMed
-
- Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras Psiquiatr. 2008;30:271–280. - PubMed
-
- Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249. - PubMed
-
- Schubart CD, Sommer IE, van Gastel WA, Goetgebuer RL, Kahn RS, Boks MP. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130:216–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

