Small heat shock protein IbpB acts as a robust chaperone in living cells by hierarchically activating its multi-type substrate-binding residues
- PMID: 23486475
- PMCID: PMC3636877
- DOI: 10.1074/jbc.M113.450437
Small heat shock protein IbpB acts as a robust chaperone in living cells by hierarchically activating its multi-type substrate-binding residues
Abstract
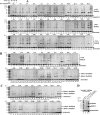
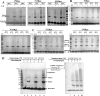
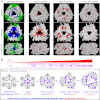
As ubiquitous molecular chaperones, small heat shock proteins (sHSPs) are crucial for protein homeostasis. It is not clear why sHSPs are able to bind a wide spectrum of non-native substrate proteins and how such binding is enhanced by heat shock. Here, by utilizing a genetically incorporated photo-cross-linker (p-benzoyl-l-phenylalanine), we systematically characterized the substrate-binding residues in IbpB (a sHSP from Escherichia coli) in living cells over a wide spectrum of temperatures (from 20 to 50 °C). A total of 20 and 48 residues were identified at normal and heat shock temperatures, respectively. They are not necessarily hydrophobic and can be classified into three types: types I and II were activated at low and normal temperatures, respectively, and type III mediated oligomerization at low temperature but switched to substrate binding at heat shock temperature. In addition, substrate binding of IbpB in living cells began at temperatures as low as 25 °C and was further enhanced upon temperature elevation. Together, these in vivo data provide novel structural insights into the wide substrate spectrum of sHSPs and suggest that sHSP is able to hierarchically activate its multi-type substrate-binding residues and thus act as a robust chaperone in cells under fluctuating growth conditions.
Figures



Similar articles
-
In vivo substrate diversity and preference of small heat shock protein IbpB as revealed by using a genetically incorporated photo-cross-linker.J Biol Chem. 2013 Nov 1;288(44):31646-54. doi: 10.1074/jbc.M113.501817. Epub 2013 Sep 17. J Biol Chem. 2013. PMID: 24045939 Free PMC article.
-
Differential degradation for small heat shock proteins IbpA and IbpB is synchronized in Escherichia coli: implications for their functional cooperation in substrate refolding.Biochem Biophys Res Commun. 2014 Sep 26;452(3):402-7. doi: 10.1016/j.bbrc.2014.08.084. Epub 2014 Aug 28. Biochem Biophys Res Commun. 2014. PMID: 25173932
-
The essential role of the flexible termini in the temperature-responsiveness of the oligomeric state and chaperone-like activity for the polydisperse small heat shock protein IbpB from Escherichia coli.J Mol Biol. 2005 Apr 8;347(4):871-84. doi: 10.1016/j.jmb.2005.01.029. J Mol Biol. 2005. PMID: 15769476
-
Chaperone function and mechanism of small heat-shock proteins.Acta Biochim Biophys Sin (Shanghai). 2014 May;46(5):347-56. doi: 10.1093/abbs/gmt152. Epub 2014 Jan 20. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24449783 Review.
-
A review on oligomeric polydispersity and oligomers-dependent holding chaperone activity of the small heat-shock protein IbpB of Escherichia coli.Cell Stress Chaperones. 2023 Nov;28(6):689-696. doi: 10.1007/s12192-023-01392-3. Epub 2023 Nov 1. Cell Stress Chaperones. 2023. PMID: 37910345 Free PMC article. Review.
Cited by
-
Biogenesis, quality control, and structural dynamics of proteins as explored in living cells via site-directed photocrosslinking.Protein Sci. 2019 Jul;28(7):1194-1209. doi: 10.1002/pro.3627. Epub 2019 May 10. Protein Sci. 2019. PMID: 31002747 Free PMC article. Review.
-
Membrane translocation process revealed by in situ structures of type II secretion system secretins.Nat Commun. 2023 Jul 7;14(1):4025. doi: 10.1038/s41467-023-39583-2. Nat Commun. 2023. PMID: 37419909 Free PMC article.
-
A Supercomplex Spanning the Inner and Outer Membranes Mediates the Biogenesis of β-Barrel Outer Membrane Proteins in Bacteria.J Biol Chem. 2016 Aug 5;291(32):16720-9. doi: 10.1074/jbc.M115.710715. Epub 2016 Jun 13. J Biol Chem. 2016. PMID: 27298319 Free PMC article.
-
The Caenorhabditis elegans 12-kDa small heat shock proteins with little in vitro chaperone activity play crucial roles for its dauer formation, longevity, and reproduction.Protein Sci. 2021 Oct;30(10):2170-2182. doi: 10.1002/pro.4160. Epub 2021 Jul 31. Protein Sci. 2021. PMID: 34272907 Free PMC article.
-
A novel mechanism for small heat shock proteins to function as molecular chaperones.Sci Rep. 2015 Mar 6;5:8811. doi: 10.1038/srep08811. Sci Rep. 2015. PMID: 25744691 Free PMC article.
References
-
- Hartl F. U., Bracher A., Hayer-Hartl M. (2011) Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 - PubMed
-
- de Jong W. W., Leunissen J. A., Voorter C. E. (1993) Evolution of the α-crystallin/small heat-shock protein family. Mol. Biol. Evol. 10, 103–126 - PubMed
-
- Basha E., Lee G. J., Breci L. A., Hausrath A. C., Buan N. R., Giese K. C., Vierling E. (2004) The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J. Biol. Chem. 279, 7566–7575 - PubMed
-
- Welker S., Rudolph B., Frenzel E., Hagn F., Liebisch G., Schmitz G., Scheuring J., Kerth A., Blume A., Weinkauf S., Haslbeck M., Kessler H., Buchner J. (2010) Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol. Cell 39, 507–520 - PubMed
-
- Török Z., Goloubinoff P., Horváth I., Tsvetkova N. M., Glatz A., Balogh G., Varvasovszki V., Los D. A., Vierling E., Crowe J. H., Vígh L. (2001) Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. U.S.A. 98, 3098–3103 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

