Essential calcium-binding cluster of Leptospira LipL32 protein for inflammatory responses through the Toll-like receptor 2 pathway
- PMID: 23486465
- PMCID: PMC3636917
- DOI: 10.1074/jbc.M112.418699
Essential calcium-binding cluster of Leptospira LipL32 protein for inflammatory responses through the Toll-like receptor 2 pathway
Abstract
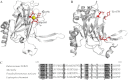
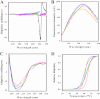
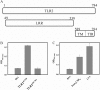
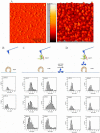
Leptospirosis is the most widespread zoonosis caused by the pathogenic Leptospira worldwide. LipL32, a 32-kDa lipoprotein, is the most abundant protein on the outer membrane of Leptospira and has an atypical poly(Asp) motif ((161)DDDDDGDD(168)). The x-ray crystallographic structure of LipL32 revealed that the calcium-binding cluster of LipL32 includes several essential residues Asp(132), Thr(133), Asp(164), Asp(165), and Tyr(178). The goals of this study were to determine possible roles of the Ca(2+)-binding cluster for the interaction of LipL32 and Toll-like receptor 2 (TLR2) in induced inflammatory responses of human kidney cells. Site-directed mutagenesis was employed to individually mutate Ca(2+)-binding residues of LipL32 to Ala, and their effects subsequently were observed. These mutations abolished primarily the structural integrity of the calcium-binding cluster in LipL32. The binding assay and atomic force microscopy analysis further demonstrated the decreased binding capability of LipL32 mutants to TLR2. Inflammatory responses induced by LipL32 variants, as determined by TLR2 pathway intermediates hCXCL8/IL-8, hCCL2/MCP-1, hMMP7, and hTNF-α, were also lessened. In conclusion, the calcium-binding cluster of LipL32 plays essential roles in presumably sustaining LipL32 conformation for its proper association with TLR2 to elicit inflammatory responses in human renal cells.
Figures
Similar articles
-
Leptospiral outer membrane lipoprotein LipL32 binding on toll-like receptor 2 of renal cells as determined with an atomic force microscope.Biochemistry. 2010 Jul 6;49(26):5408-17. doi: 10.1021/bi100058w. Biochemistry. 2010. PMID: 20513152
-
Peptidoglycan mediates Leptospira outer membrane protein Loa22 to toll-like receptor 2 for inflammatory interaction: a novel innate immune recognition.Sci Rep. 2021 Jan 13;11(1):1064. doi: 10.1038/s41598-020-79662-8. Sci Rep. 2021. PMID: 33441663 Free PMC article.
-
Active Components of Leptospira Outer Membrane Protein LipL32 to Toll-Like Receptor 2.Sci Rep. 2017 Aug 21;7(1):8363. doi: 10.1038/s41598-017-08743-y. Sci Rep. 2017. PMID: 28827637 Free PMC article.
-
The lipoprotein LipL32, an enigma of leptospiral biology.Vet Microbiol. 2013 Mar 23;162(2-4):305-314. doi: 10.1016/j.vetmic.2012.11.005. Epub 2012 Nov 9. Vet Microbiol. 2013. PMID: 23206414 Review.
-
Leptospirosis: a Toll road from B lymphocytes.Chang Gung Med J. 2010 Nov-Dec;33(6):591-601. Chang Gung Med J. 2010. PMID: 21199604 Review.
Cited by
-
Leptospiral adhesins: from identification to future perspectives.Front Microbiol. 2024 Aug 13;15:1458655. doi: 10.3389/fmicb.2024.1458655. eCollection 2024. Front Microbiol. 2024. PMID: 39206373 Free PMC article. Review.
-
Leptospiral outer membrane protein LipL32 induces inflammation and kidney injury in zebrafish larvae.Sci Rep. 2016 Jun 9;6:27838. doi: 10.1038/srep27838. Sci Rep. 2016. PMID: 27278903 Free PMC article.
-
Micronutrients and Leptospirosis: A Review of the Current Evidence.PLoS Negl Trop Dis. 2016 Jul 7;10(7):e0004652. doi: 10.1371/journal.pntd.0004652. eCollection 2016 Jul. PLoS Negl Trop Dis. 2016. PMID: 27387046 Free PMC article. Review.
-
The Cross-Talk between Spirochetal Lipoproteins and Immunity.Front Immunol. 2014 Jun 30;5:310. doi: 10.3389/fimmu.2014.00310. eCollection 2014. Front Immunol. 2014. PMID: 25071771 Free PMC article. Review.
-
Regulation of H+-pyrophosphatase by 14-3-3 Proteins from Arabidopsis thaliana.J Membr Biol. 2018 Apr;251(2):263-276. doi: 10.1007/s00232-018-0020-4. Epub 2018 Feb 16. J Membr Biol. 2018. PMID: 29453559
References
-
- Yang C. W. (2007) Leptospirosis renal disease: understanding the initiation by Toll-like receptors. Kidney Int. 72, 918–925 - PubMed
-
- Dolhnikoff M., Mauad T., Bethlem E. P., Carvalho C. R. (2007) Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz. J. Infect. Dis. 11, 142–148 - PubMed
-
- Farr R. W. (1995) Leptospirosis. Clin. Infect. Dis. 21, 1–8 - PubMed
-
- Yang C. W., Wu M. S., Pan M. J. (2001) Leptospirosis renal disease. Nephrol. Dial. Transplant. 16, 73–77 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

