HOXC9 regulates formation of parachordal lymphangioplasts and the thoracic duct in zebrafish via stabilin 2
- PMID: 23484014
- PMCID: PMC3590145
- DOI: 10.1371/journal.pone.0058311
HOXC9 regulates formation of parachordal lymphangioplasts and the thoracic duct in zebrafish via stabilin 2
Abstract
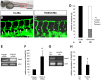
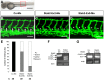
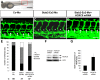
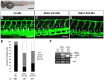
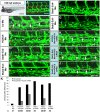
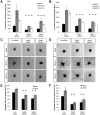
HOXC9 belongs to the family of homeobox transcription factors, which are regulators of body patterning and development. HOXC9 acts as a negative regulator on blood endothelial cells but its function on lymphatic vessel development has not been studied. The hyaluronan receptor homologs stabilin 1 and stabilin 2 are expressed in endothelial cells but their role in vascular development is poorly understood. This study was aimed at investigating the function of HOXC9, stabilin 2 and stabilin 1 in lymphatic vessel development in zebrafish and in endothelial cells. Morpholino-based expression silencing of HOXC9 repressed parachordal lymphangioblast assembly and thoracic duct formation in zebrafish. HOXC9 positively regulated stabilin 2 expression in zebrafish and in HUVECs and expression silencing of stabilin 2 phenocopied the HOXC9 morphant vascular phenotype. This effect could be compensated by HOXC9 mRNA injection in stabilin 2 morphant zebrafish embryos. Stabilin 1 also regulated parachordal lymphangioblast and thoracic duct formation in zebrafish but acts independently of HOXC9. On a cellular level stabilin 1 and stabilin 2 regulated endothelial cell migration and in-gel sprouting angiogenesis in endothelial cells. HOXC9 was identified as novel transcriptional regulator of parachordal lymphangioblast assembly and thoracic duct formation in zebrafish that acts via stabilin 2. Stabilin 1, which acts independently of HOXC9, has a similar function in zebrafish and both receptors control important cellular processes in endothelial cells.
Conflict of interest statement
Figures

Similar articles
-
The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8.Circ Res. 2011 May 27;108(11):1367-77. doi: 10.1161/CIRCRESAHA.111.244095. Epub 2011 Apr 14. Circ Res. 2011. PMID: 21493894
-
MicroRNA-126a Directs Lymphangiogenesis Through Interacting With Chemokine and Flt4 Signaling in Zebrafish.Arterioscler Thromb Vasc Biol. 2016 Dec;36(12):2381-2393. doi: 10.1161/ATVBAHA.116.308120. Epub 2016 Oct 27. Arterioscler Thromb Vasc Biol. 2016. PMID: 27789478
-
Role of synectin in lymphatic development in zebrafish and frogs.Blood. 2010 Oct 28;116(17):3356-66. doi: 10.1182/blood-2009-11-254557. Epub 2010 Jul 14. Blood. 2010. PMID: 20631376 Free PMC article.
-
HOXC9: a key regulator of endothelial cell quiescence and vascular morphogenesis.Trends Cardiovasc Med. 2012 Jan;22(1):7-11. doi: 10.1016/j.tcm.2012.06.002. Epub 2012 Jul 28. Trends Cardiovasc Med. 2012. PMID: 22841837 Review.
-
Stabilin-1, a homeostatic scavenger receptor with multiple functions.J Cell Mol Med. 2006 Jul-Sep;10(3):635-49. doi: 10.1111/j.1582-4934.2006.tb00425.x. J Cell Mol Med. 2006. PMID: 16989725 Free PMC article. Review.
Cited by
-
Recent advancements in understanding of biological role of homeobox C9 in human cancers.World J Clin Oncol. 2024 Sep 24;15(9):1168-1176. doi: 10.5306/wjco.v15.i9.1168. World J Clin Oncol. 2024. PMID: 39351453 Free PMC article. Review.
-
Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 3;9(1):9. doi: 10.1038/s41392-023-01723-x. Signal Transduct Target Ther. 2024. PMID: 38172098 Free PMC article. Review.
-
Hyaluronic acid receptor Stabilin-2 regulates Erk phosphorylation and arterial--venous differentiation in zebrafish.PLoS One. 2014 Feb 28;9(2):e88614. doi: 10.1371/journal.pone.0088614. eCollection 2014. PLoS One. 2014. PMID: 24586357 Free PMC article.
-
Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake.ACS Nano. 2018 Mar 27;12(3):2138-2150. doi: 10.1021/acsnano.7b06995. Epub 2018 Jan 18. ACS Nano. 2018. PMID: 29320626 Free PMC article.
-
The bipartite rac1 Guanine nucleotide exchange factor engulfment and cell motility 1/dedicator of cytokinesis 180 (elmo1/dock180) protects endothelial cells from apoptosis in blood vessel development.J Biol Chem. 2015 Mar 6;290(10):6408-18. doi: 10.1074/jbc.M114.633701. Epub 2015 Jan 13. J Biol Chem. 2015. PMID: 25586182 Free PMC article.
References
-
- Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478. - PubMed
-
- Tammela T, Alitalo K (2010) Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140: 460–476. - PubMed
-
- Shah N, Sukumar S (2010) The Hox genes and their roles in oncogenesis. Nat Rev Cancer 10: 361–371. - PubMed
-
- Cantile M, Schiavo G, Terracciano L, Cillo C (2008) Homeobox genes in normal and abnormal vasculogenesis. Nutr Metab Cardiovasc Dis 18: 651–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

